Naming alkenes follows the same rules we discussed earlier for the IUPAC nomenclature rules for alkanes.
This is a brief summary of the steps:
Step 1. Identify the parent chain.
Step 2. Identify the substituents.
Step 3. Number the parent chain.
Step 4. Put everything together, having the substituents in alphabetical order.
And this is an example of naming an alkane:

There are two main differences you need to consider when naming alkenes:
1) The longest chain must include the π bond.
2) Change the suffix in the parent chain from “ane” to “ene”.
The latest IUPAC recommendation is to place the locant before the suffix “ene.” However, both options are acceptable (see below).
Let’s see how these modifications affect naming the following alkane and alkene:

Notice that the parent chain changes from nonane to octane because it is only possible to include the c=c double bond in an eight-carbon continuous chain.
And the second change is the numbering. Even though starting from left would place the methyl group at position two, the c=c bond in that case would have been at position 7, which is incorrect:

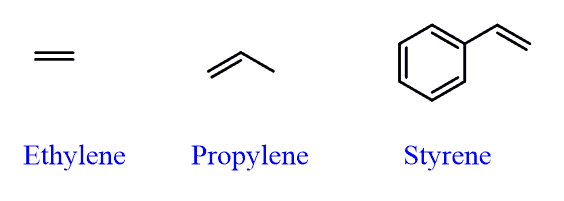
There are also common names for some simple alkenes shown below:
Naming Cyclic Alkenes
The same rules apply when a cyclic alkene is named. The prefix “cyclo’ combined with the suffix “ene” is the only addition here:

Notice that the numbering starts from the double bond and goes such that it includes both carbons and gives substituents the lowest possible numbers. Notice that because the parent is cyclohexene, we may also omit the locant 1-ene because it is assumed to be between C1 and C2. This means, however, you need to make sure the locant for the alkyl group is assigned correctly by placing the double bond between C1 and C2. For example, you cannot start numbering from the top C=C carbon and go counterclockwise, giving locant 3 for the methyl group, because in that case, the double bond would be between carbon 1 and 6:

Naming Cis and Trans Alkenes
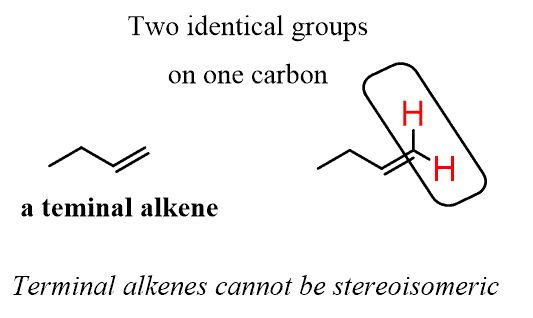
In the first example of naming alkenes, we used a molecule where the double bond was in the terminal position. These are called terminal alkenes, and because one of the carbons is connected to two hydrogens, they are not stereoisomeric, i.e., they cannot be cis or trans:
Internal alkenes, on the other hand, can be cis or trans depending on the relative position of two identical alkyl groups on both carbons of the double bond.
For example, 3-hexene can have two ethyl groups either on the same side of the double bond or on opposite sides of the double bond:
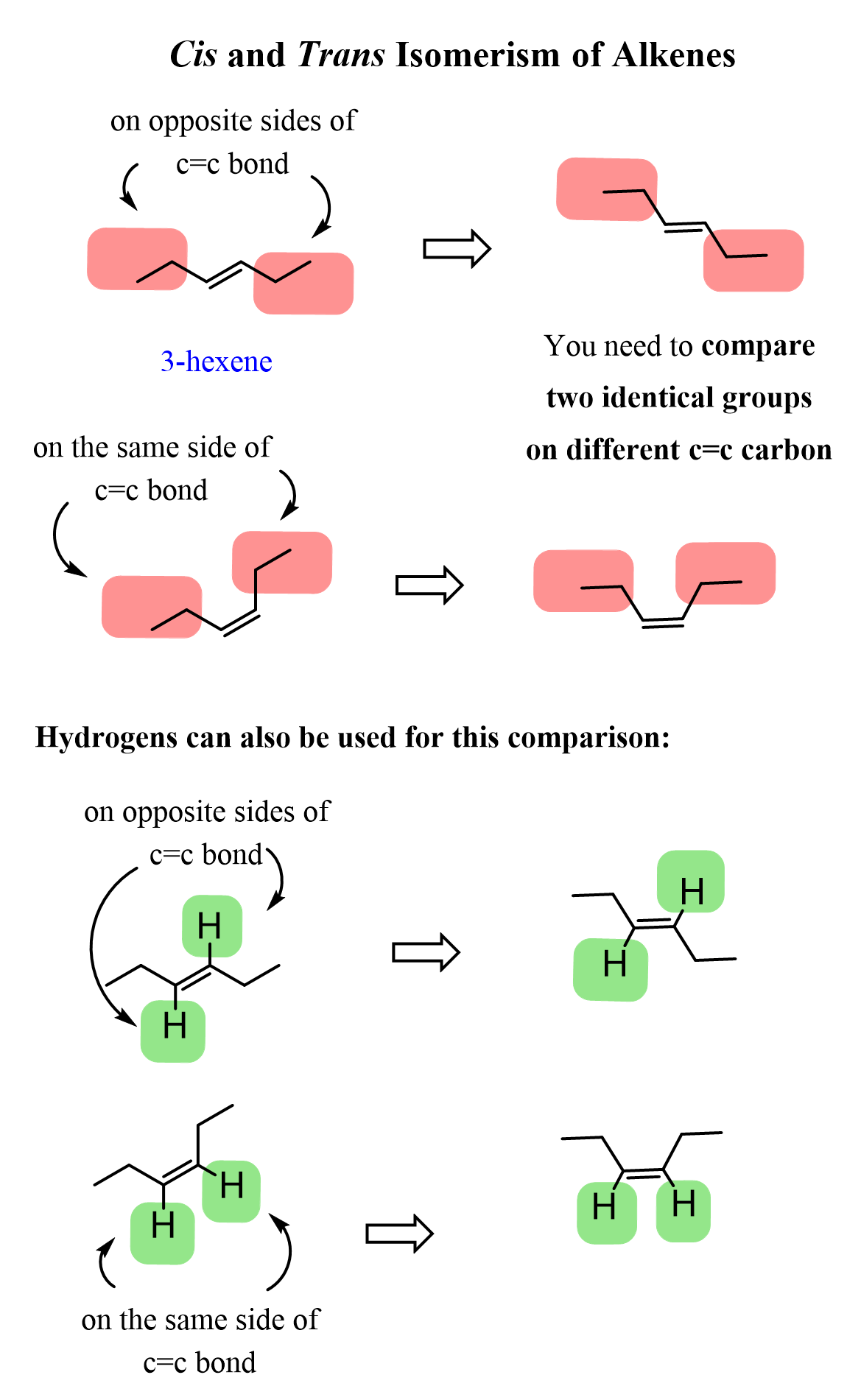
Therefore, when naming it, the cis and trans designation is used by placing them right before the main part.
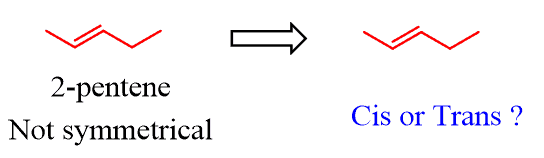
The cis and trans designation is not used based on alkyl groups only.
For example, 2-pentene is not a symmetrical molecule; thus, we cannot have two identical alkyl groups on both carbons of the c=c bond:
However, remember that there are hydrogens that are not shown since it is a bond-line structure:
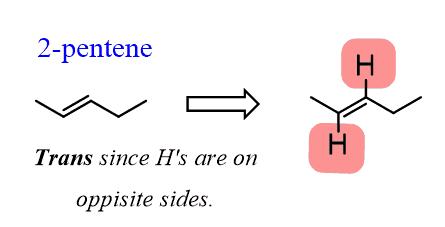
If we draw out these hydrogens, it becomes evident that they can be cis or trans, just like the alkyl groups in 3-hexene:
Naming E and Z Alkenes
The cis and trans designation works only for alkenes with two identical groups on the two carbons of the double bond.
To illustrate this limitation, let’s consider two isomeric alkenes having four different groups on the double bond:

These two are not identical compounds; they are stereoisomers-specifically diastereomers. The question is how to distinguish them by their names. For this, the E and Z designation is used. It is a topic worth a separate blog post; however, as a reminder, the priorities are assigned following the same rules for the R and S configuration.
For example, to name the first alkene, you need to first name it according to the IUPAC rules we discussed earlier:
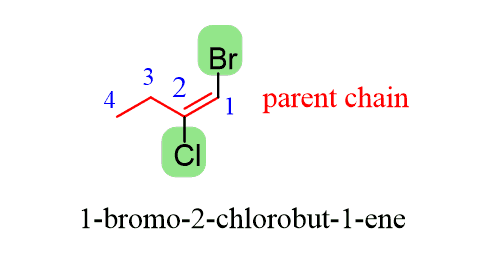
In addition to this, you need to figure out that it is a stereoisomeric alkene (i.e., it can be cis/trans or E/Z).
And, depending on its stereochemistry, you put the corresponding designation before the name.
In this case, we have an E alkene since the two Cl and ethyl groups are the higher priorities on each carbon and they are on opposite sides of the double bond:
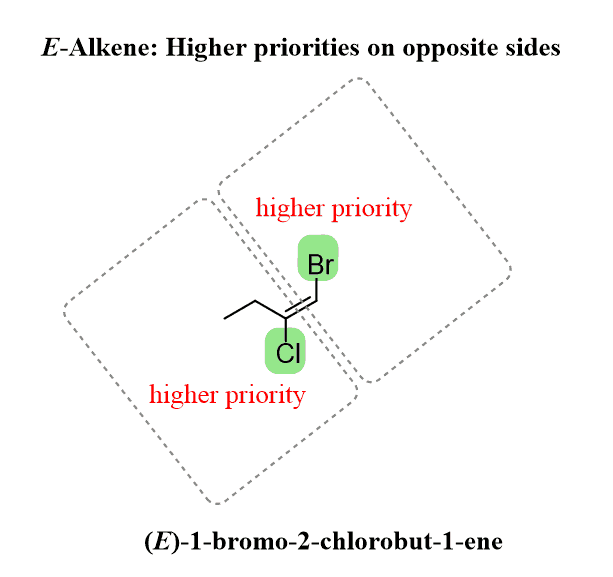
If two stereoisomeric double bonds are present in the molecule, then the E/Z designation is specified for each alkene:
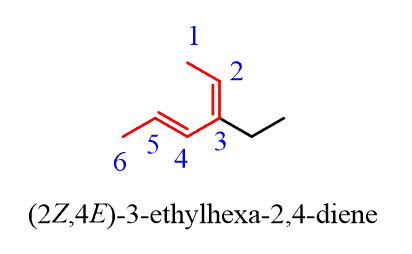
Notice also that the suffix changes from “-ene” to “diene”.
IUPAC Moments
IUPAC now says that…

Quoting the IUPAC 2013 Blue Book:
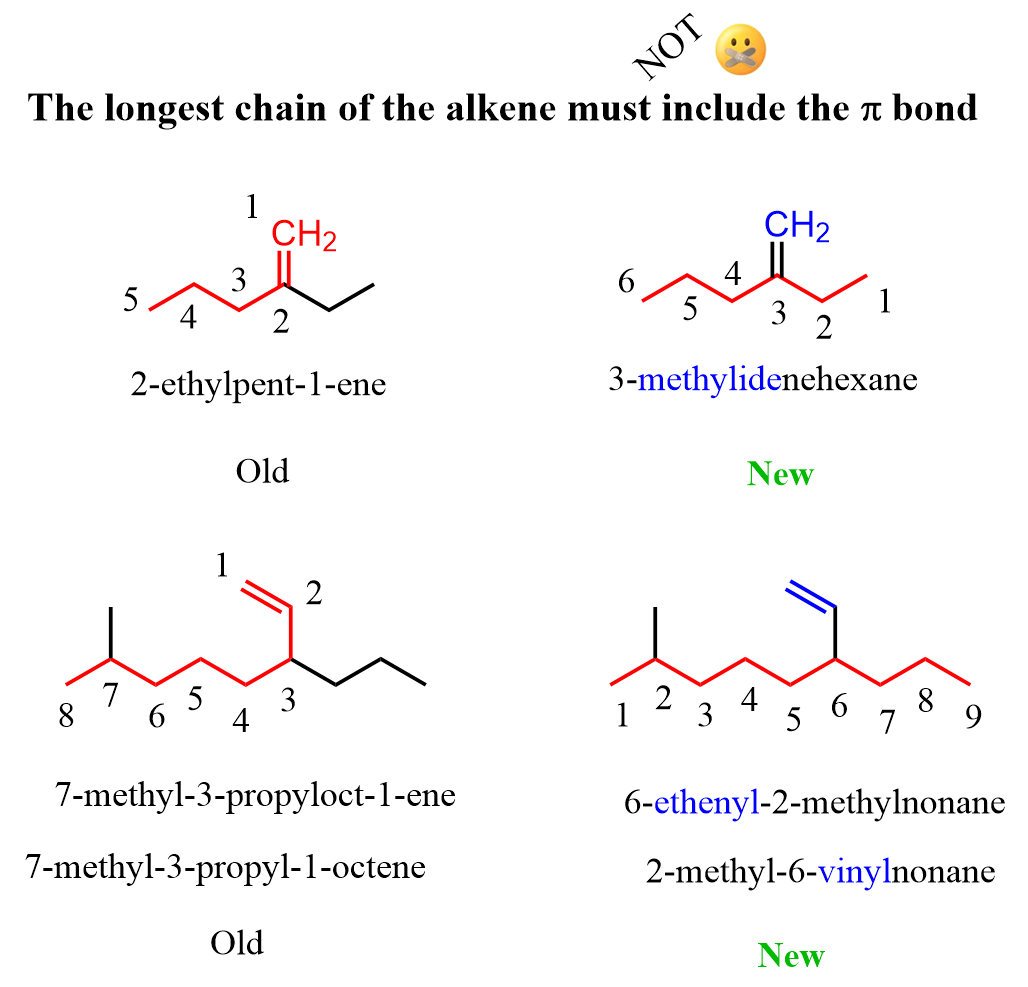
P-44.3 – Unsaturation The parent chain of an alkene must include the double bond. This rule has been modified from previous rules; seniority is now given to the length of the chain rather than to unsaturation.
For example, based on the new rules, this molecule is named 3-mehylidenehexane rather than 2-ethylpent-1-ene:
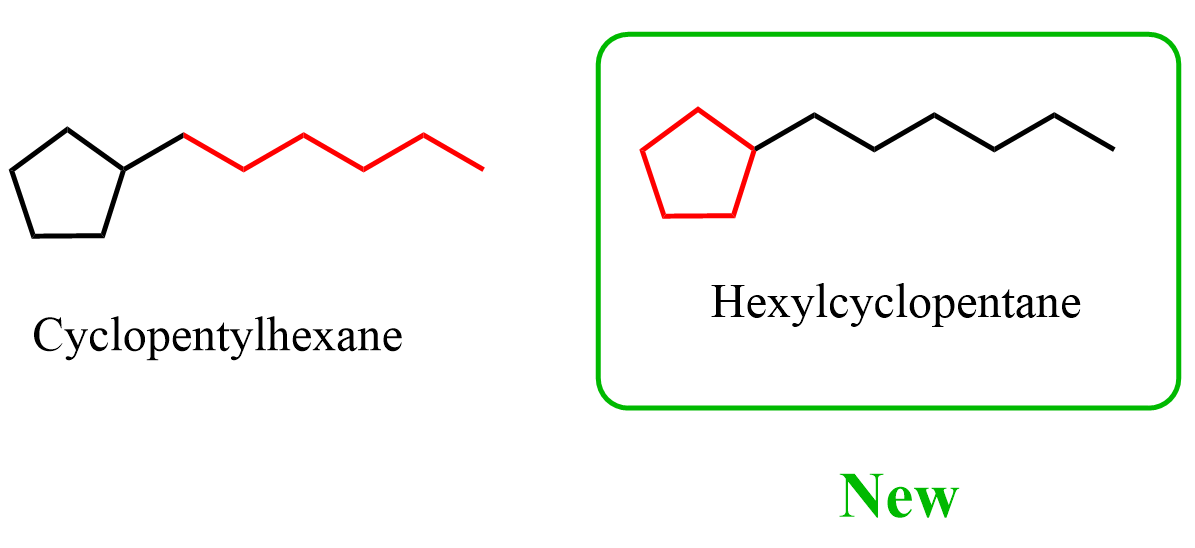
P-44.1.2.2 – Rings Systems composed of rings and chains (exclusive of linear phanes) Two methods are recognized to name systems composed of rings and chains (exclusive of linear phanes). (1) Within the same class, a ring or ring system has seniority over a chain. When a ring and a chain contain the same senior element, the ring is chosen as the parent. Rings and chains are chosen regardless of their degree of hydrogenation. As a consequence, this approach prefers the choice of a ring over a chain in systems composed of cyclic and acyclic hydrocarbons.
So, apparently, hexylcyclopentane is the preferred IUPAC name (PIN) for what we called cyclopentylhexane:
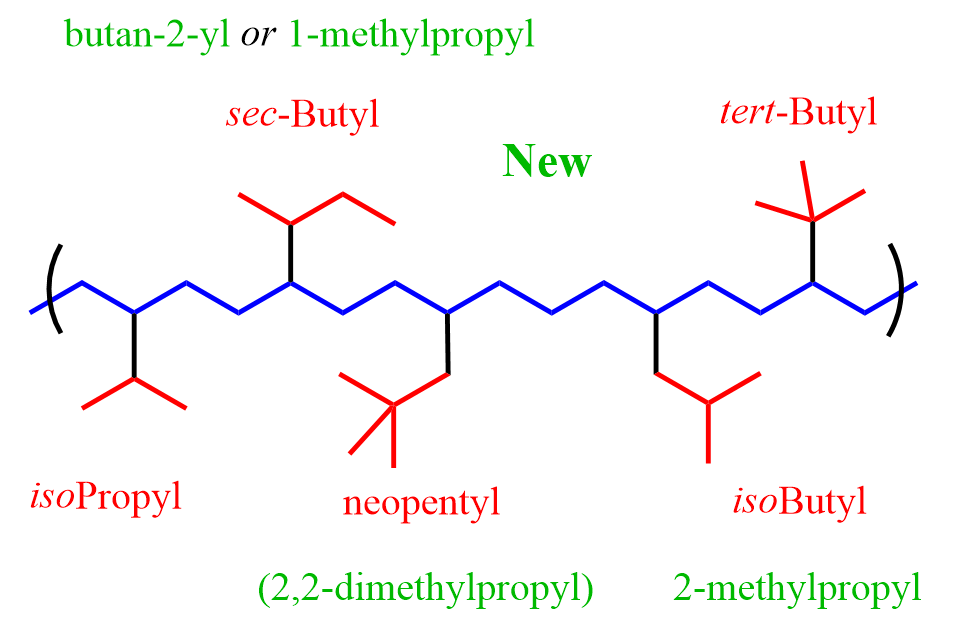
P-57.1.4 – Substitutents Retained prefixes no longer recommended
The retained names phenethyl (2-phenylethyl) for C6H5-CH2-CH2–; benzhydryl (diphenylmethyl), for (C6H5)2CH–; isobutyl (2-methylpropyl) for (CH3)2CH-CH2–; sec-butyl (butan-2-yl, 1-methylpropyl) for CH3-CH2-CH(CH3)–; isopentyl (3-methylbutyl) for (CH3)2CH-CH2-CH2–; tert-pentyl (2-methylbutan-2-yl, 1,1-dimethylpropyl) for CH3-CH2-C(CH3)2–; and neopentyl (2,2-dimethylpropyl) for (CH3)3C-CH2–; are no longer recommended; the first name in parentheses is the preferred prefix name.
So, which rules do we follow when naming certain alkanes, cycloalkeanes, and alkenes? Honesty, I think this is not the most important part of learning and understanding organic chemistry… In most of the examples and practice problems at Chemistry Steps, we follow the earlier rules, as this is what most textbooks, at least in the US, do. Ultimately, it is up to your instructor how they want to address this, so follow the rules given in the class and double-check for clarification if needed.
Check this 69-question, Multiple-Choice Quiz with a 2-hour Video Solution on naming alkanes, alkyl halides, alkenes, alkynes, aromatic compounds, alcohols, aldehydes, ketones, and compounds containing multiple functional groups.
IUPAC Nomenclature Summary Quiz
Alkanes and Cycloalkanes Practice Quiz
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Nomenclature of Alkyl Halides
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides





Shouldn’t problem A be trans instead of using E. It has two identical groups on the two carbons on the double bond.
Hello,
You can use the cis and trans notation when applicabale but it doesn’t exclude or make the use of e/z nomenclature wrong. Quite the opposite, the e/z system is more comprehensive and importantly, it describes the absolute configuration of the double bond, while the cis and trans are referred to the relative configuration.
Thank you for the explanation, much appreciated!
I think problem G should be Hex (6), not Hept (7)
Yes, that is correct. It is definitely not “hep” anyways. Fixed.
I can’t View the answer for H
Hello,
Looks like you don’t have an account, do you?
Hiii
Can you please upload more practice questions based on these topic ??
Hello,
You can check out the summary quiz on IUPAC nomenclature which covers naming alkanes, alkenes, alkynes, aromatic compounds, alcohols, aldehydes, ketones, and compounds containing multiple functional groups. It comes with a 2-hour video solution.
IUPAC Nomenclature – a Summary Quiz
Could question F also be (z)-2-bromo-4-isopropylhex-2-ene?
Hi,
Don’t forget that given the same number of atoms, you need to choose a parent chain with as many substituents as possible. In this case, you would only have one (isopropyl), while the correct option has two (ethyl and methyl).
In option C, there is a carbon that has 5 bonds. Doesn’t carbon have a maximum valency of 4?
Where is that? If you mean in question C, there is no carbon with five bonds. And yes, carbon cannot have more than four bonds.
P.S.
If you’re just starting organic chemistry, you might want to look away… but of course, we can’t have organic chemistry without a few rule breakers. Meet methanium – a positively charged overachiever with the formula [CH₅]+ 🥇. https://en.wikipedia.org/wiki/Methanium
for D, why start numbering from the bottom (near the alkene) rather than the top which would make the substituents on a lower numbered carbon?
The double bond needs to have the lowest number even if the alkyl substituents don’t.
Pls For D when numbering why didn’t you number 5, 6 and 7 to the right instead to give u leads substituents
You numbered 5 6 and 7 to the left slightly confused
If numbering gives you two parent chains of equal length, you need to choose the one with the greater number of substituents.
You can check the post “Naming Alkanes” for more details.
Thank you so much
Just a heads up – mis-spelling of Trans (as Trnas) in a heading near the top of the page.
Thanks for letting me know, but I was not able to find it. Send me a screenshot or something if you get a chance 🧐
Under the section called “Naming Cis and Trans Alkenes”, about a quarter way down through the page, right under the line that says “For example, 3-hexene can have two ethyl groups either on the same side of the double bond or on opposite sides of the double bond:”
There is a label that says “Cis and Trnas Isomerism of Alkenes”
Ah, so it was the image… Finally got that trnas 🙂 – thanks for letting me know!
Does question 3-G have a new answer since the new rule prioritizes chain length over unsaturation?
Yes, and we’ll also need to start numbering from the other end to give substituents the lowest possible locants, which will give vinyl or ethenyl on position 4.