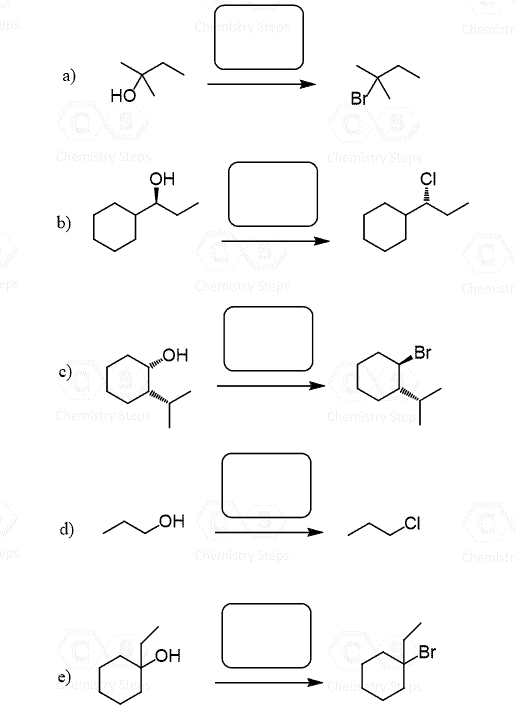
Alcohols can be converted to alkyl halides by reacting with HX (X = Cl, Br, I) acids. The reaction works for 1°, 2°, and 3° alcohols:

Let’s now understand how this happens.
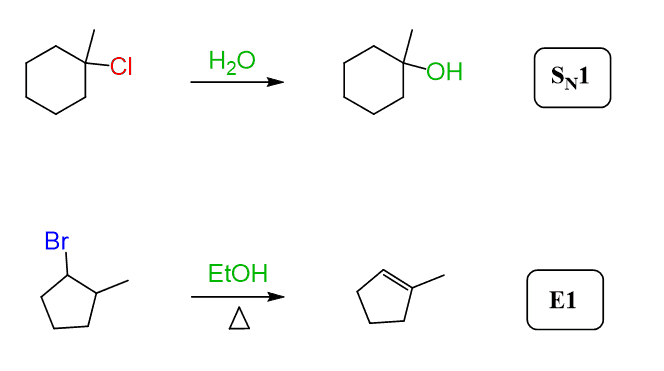
We have seen many times in SN1 and E1 reactions that alcohols can serve as weak nucleophiles and weak bases when reacted with alkyl halides:
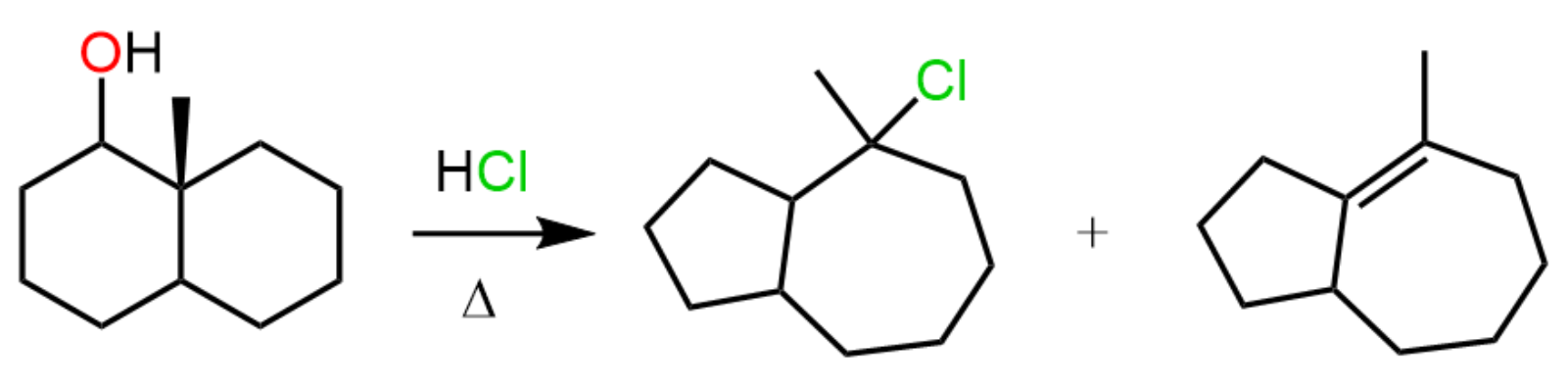
The first reaction of going from an alkyl halide to an alcohol is the reversed reaction of converting alcohols to alkyl halides, and you might have a good question to ask here – how do you control the outcome of the reaction?
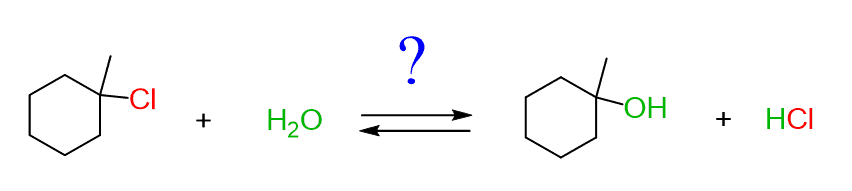
And the answer is that it is all about the concentration of the reactants. If you need to convert the alcohol to an alkyl halide, you’d add a lot of acid, and if you need to prepare an alcohol from the alkyl halide you add a lot of water or hydroxide, depending on what substrate you are using.
Aside from this, the equilibrium can be shifted towards the products by removing one of the products, for example, by distillation.
In this post, we will focus on the conversion of alcohols to alkyl halides, as the opposite reaction(s) are covered in the SN1 and SN2 reactions.
What is the Mechanism of converting alcohols to alkyl halides?
We are working with a substitution – the OH group is replaced with a halogen. Therefore, regardless of what alcohol is used (1o, 2o, or 3o), the principle of this substitution is to convert the OH into a good leaving group and kick it out by the halide ion (SN2) or the nucleophile may attack after the OH departs as a leaving group, which would correspond to an SN1 process:
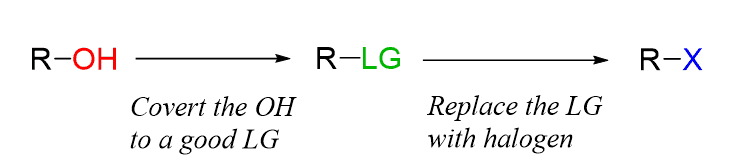
For both mechanisms, the first step is the protonation of the alcohol to create the good leaving group H2O, after which the nucleophilic substitution occurs.
There are some differences in the mechanism of the substitution depending on the type of alcohol. Let’s discuss them separately.
Reaction of HX acids with Methyl and Primary Alcohols
Methyl and primary alcohols are converted to alkyl halides via SN2. The I– and Br– are good nucleophiles and attack the carbon, kicking out the +OH2 in the form of neutral water molecule.

HCl works fine as well; however, it is not as strong an acid, and the chloride ion is not a great nucleophile in these conditions. Thus, ZnCl2 is sometimes added as a catalyst to speed up the reaction.
The Reaction of HX Acids with Secondary Alcohols
Secondary alcohols can undergo SN2 and SN1 reactions:

The problem with the SN1 mechanism is the possible rearrangements for certain alcohols, as well as the racemization if the carbon atom is a chiral center:
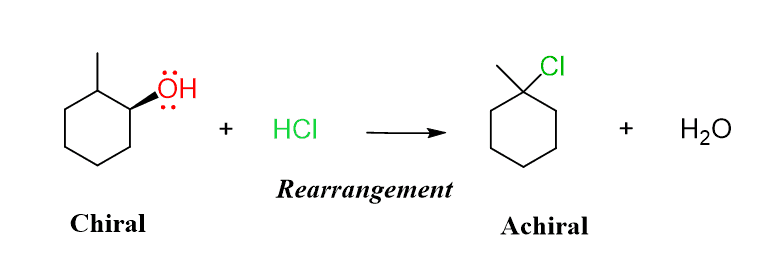
At this point, we can see the two main disadvantages of converting alcohols to alkyl halides using hydrogen halides:
1) The strong acidic conditions are often not suitable for organic molecules.
2) The SN1 mechanism lacks the stereochemical control, and rearrangements add another liability as the regiochemical outcome of the reaction.
For this, there are alternative methods such as the use of SOCl2, PBr3, and converting alcohols to sulfonyl esters like mesylates and tosylates.
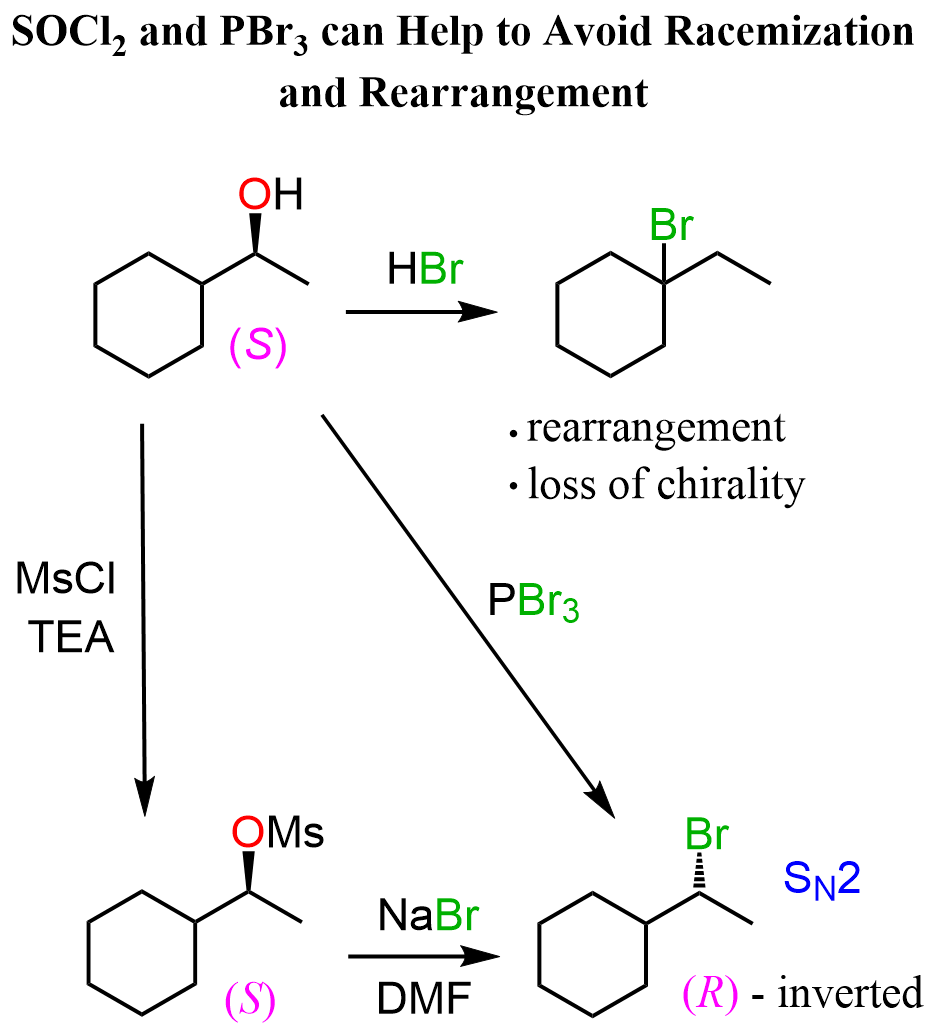
Both methods are covered in separate posts, as there is too much to talk about in one article:
SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
Mesylates and Tosylates as Good Leaving Groups
Reaction of HX acids with Tertiary Alcohols
Tertiary alcohols work the best for acid-catalyzed conversion to alkyl halides. The reaction goes by an SN1 mechanism, but the carbocation is “very” stable and there is usually no problem of a rearrangement:

Check out this 65-question, Multiple-Choice Quiz with a 3-hour Video Solution covering Nucleophilic Substitution and Elimination Reactions:
Nucleophilic Substitution and Elimination Practice Quiz
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides