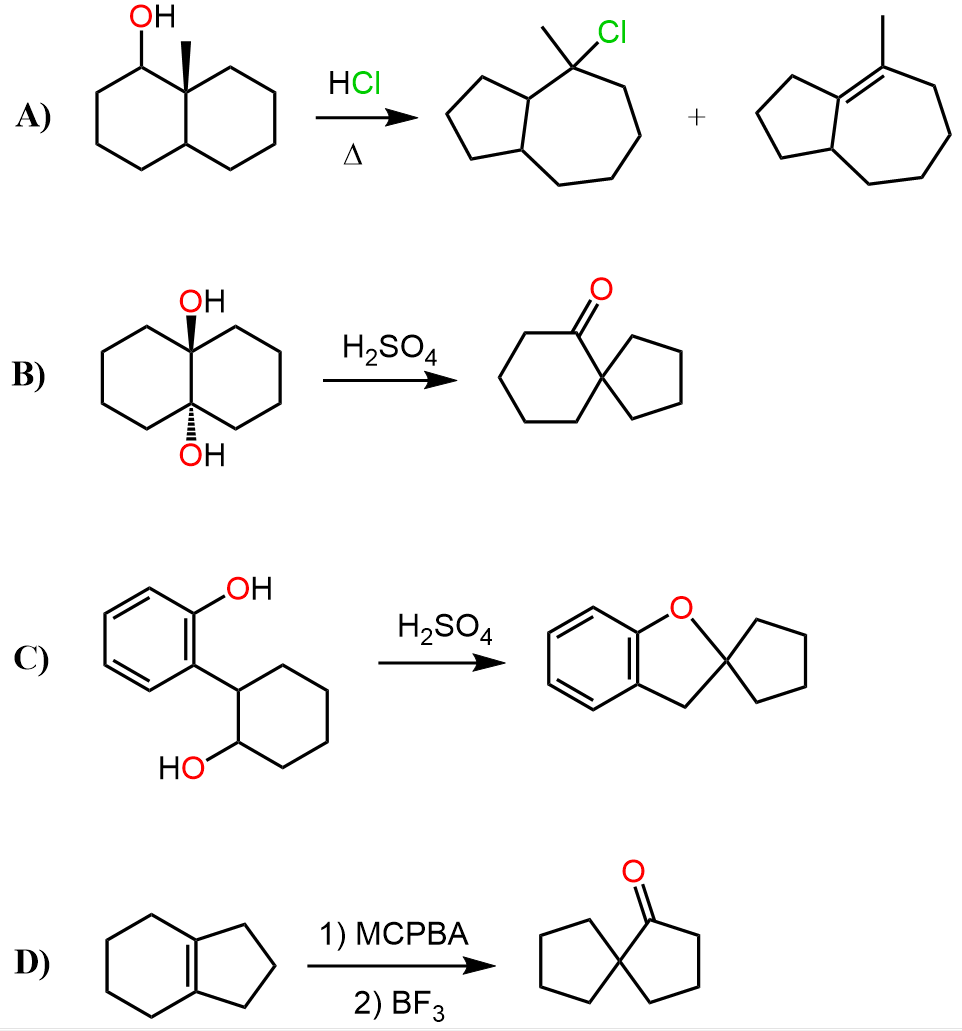
In the previous post, we discussed ring expansion rearrangements where 4 and 5-membered rings were transformed into more stable larger rings.
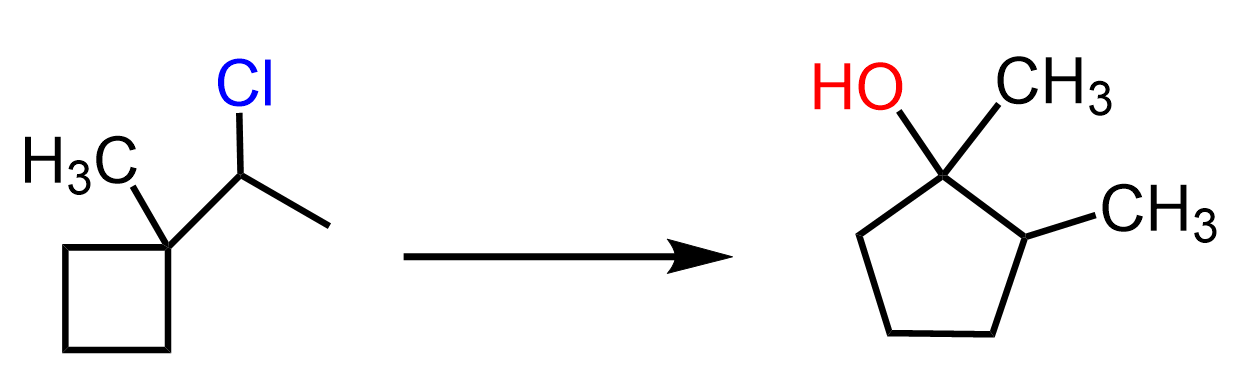
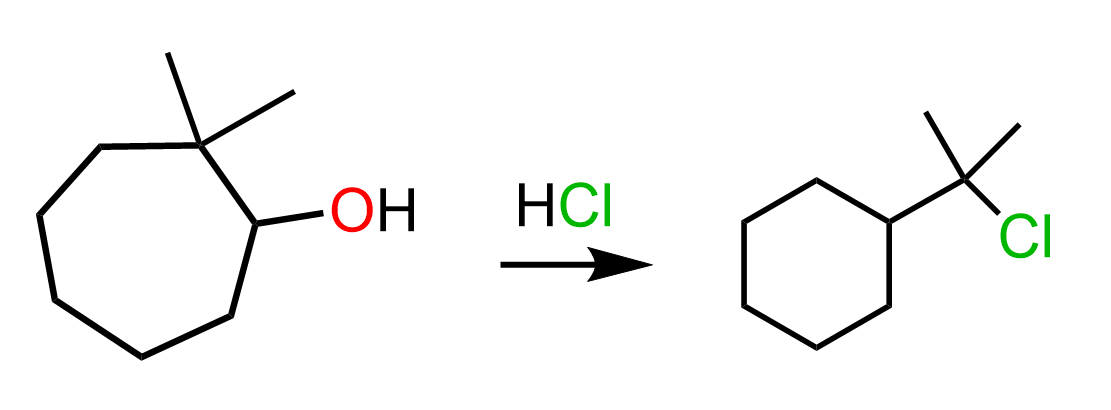
Sometimes, the process goes in reverse direction, and larger rings are transformed into smaller rings. These are called ring contraction rearrangements. For example, when the following cycloheptyl alcohol is treated with hydrochloric acid, a six-membered ring is formed:
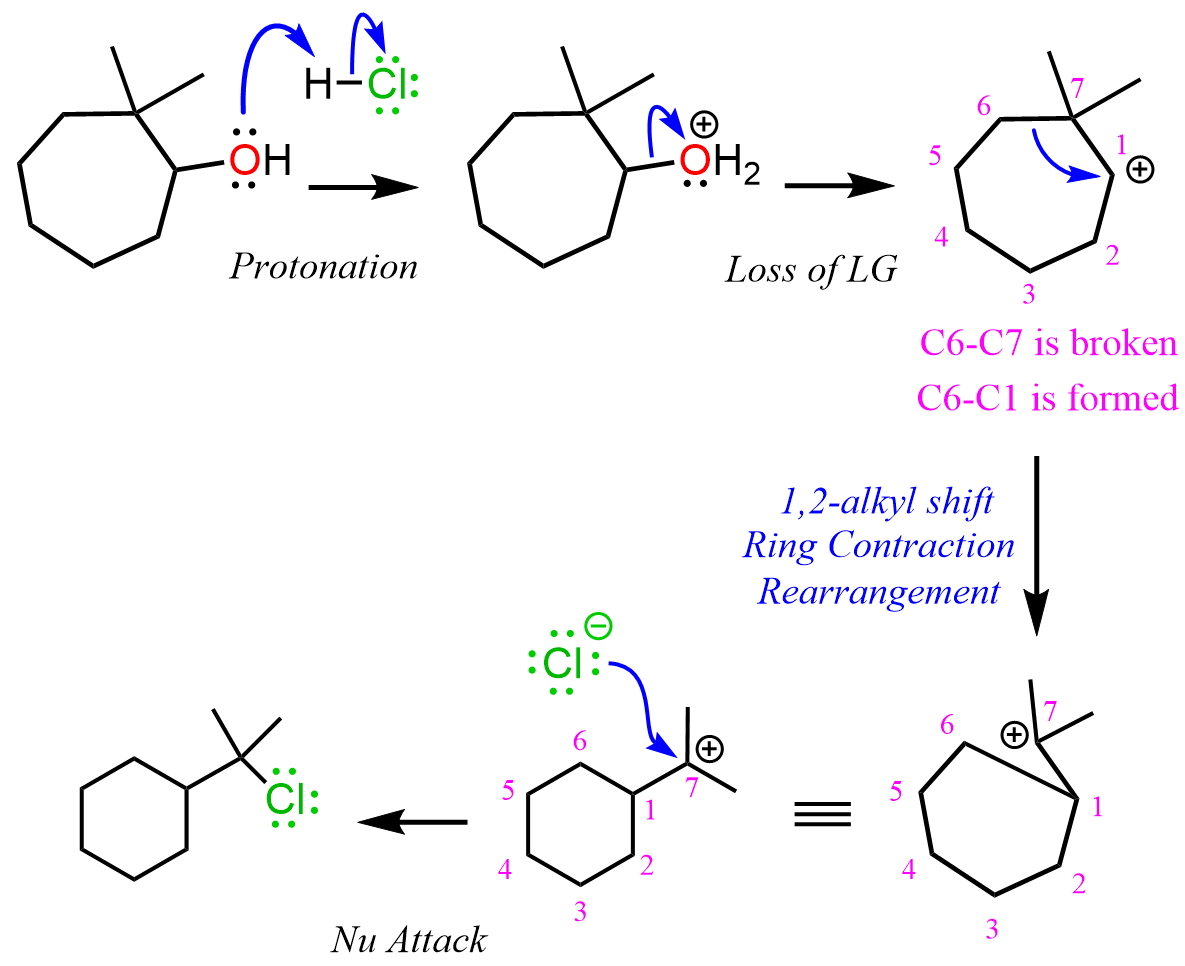
We have seen many times that the reaction of alcohols with acids starts with a protonation of the hydroxyl group. After the loss of water, a secondary carbocation is formed which undergoes a 1,2-alkyl shift that breaks the C6-C7 bond, and the new, C6-C1 bond is formed. As a result, the seven-membered ring is transformed into a more stable six-membered ring with a tertiary carbocation that is then attacked by the chloride ion:
An interesting type of ring contraction rearrangement is the transformation of cyclohexane epoxides (cylcohexene oxides, oxiranes) to five-membered carbonyl compounds in the presence of Lewis acid catalysts:
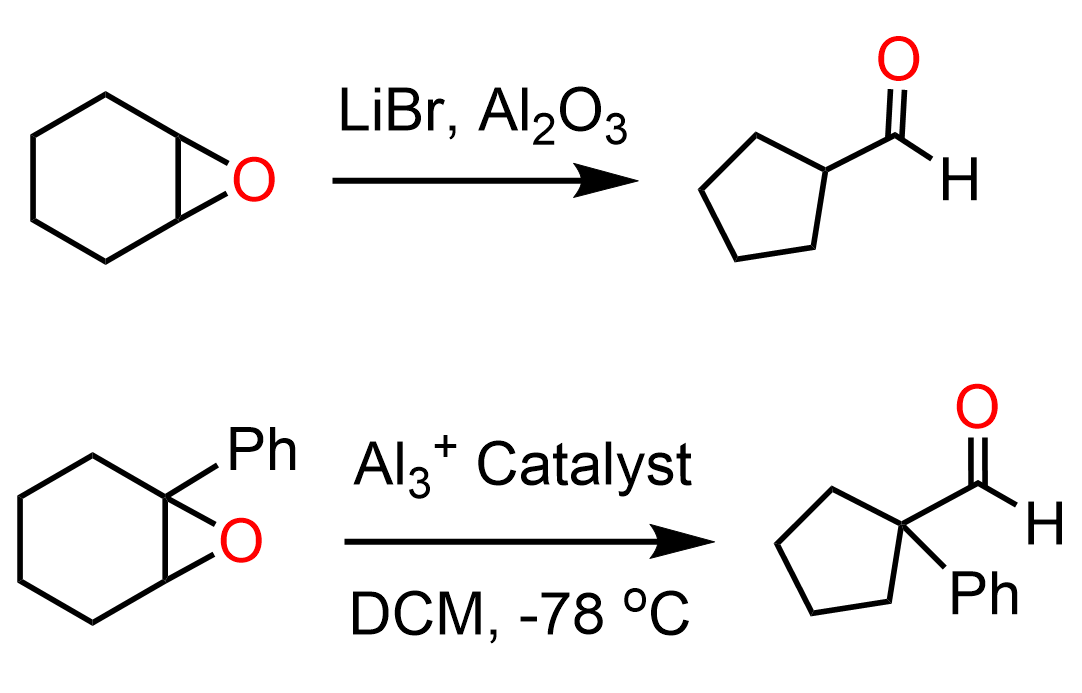
The ring contraction of epoxides is catalyzed by Lewis acids which activate the epoxide coordinating the oxygen. This is what we learn in the ring-opening reactions of epoxides under acidic conditions. Once the epoxide is opened, a carbocation is formed which undergoes a 1,2-ring contraction alkyl shift.
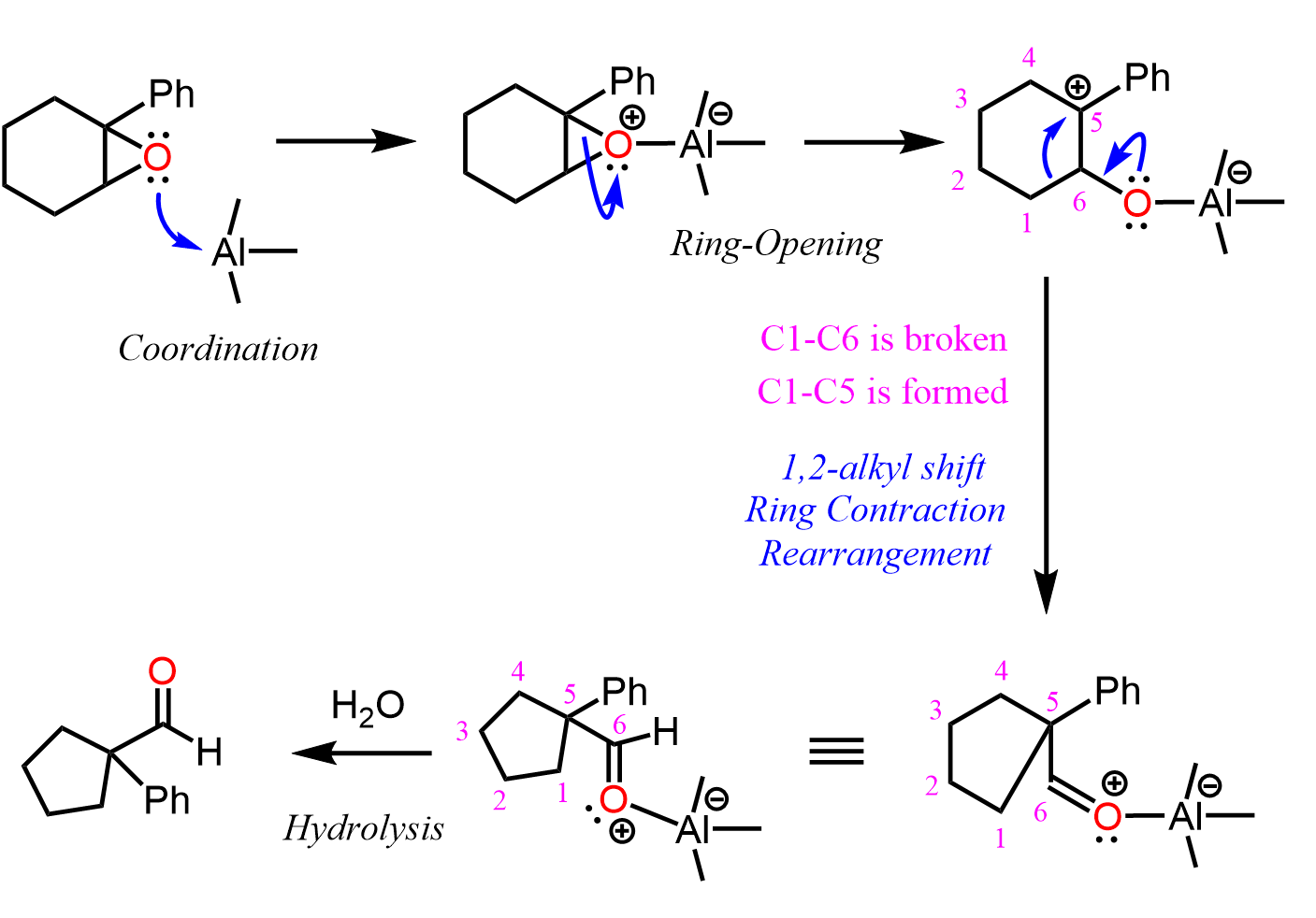
A nice review of ring contraction rearrangements including those of epoxides is summarized by L. Silva in Tetrahedron 58 (2002) 9137–9161.
Favorskii Rearrangement
If you are in the first semester of organic chemistry and the terms “enol” and “enolate” are unfamiliar to you, you can skip this part of the discussion.
Although the Favorskii rearrangement does not pertain to cyclic α-halo ketones only, these cyclic ketones undergo ring contraction rearrangements when treated with a base. Therefore, we will look at the ring contraction of 2-bromocyclohexanine.
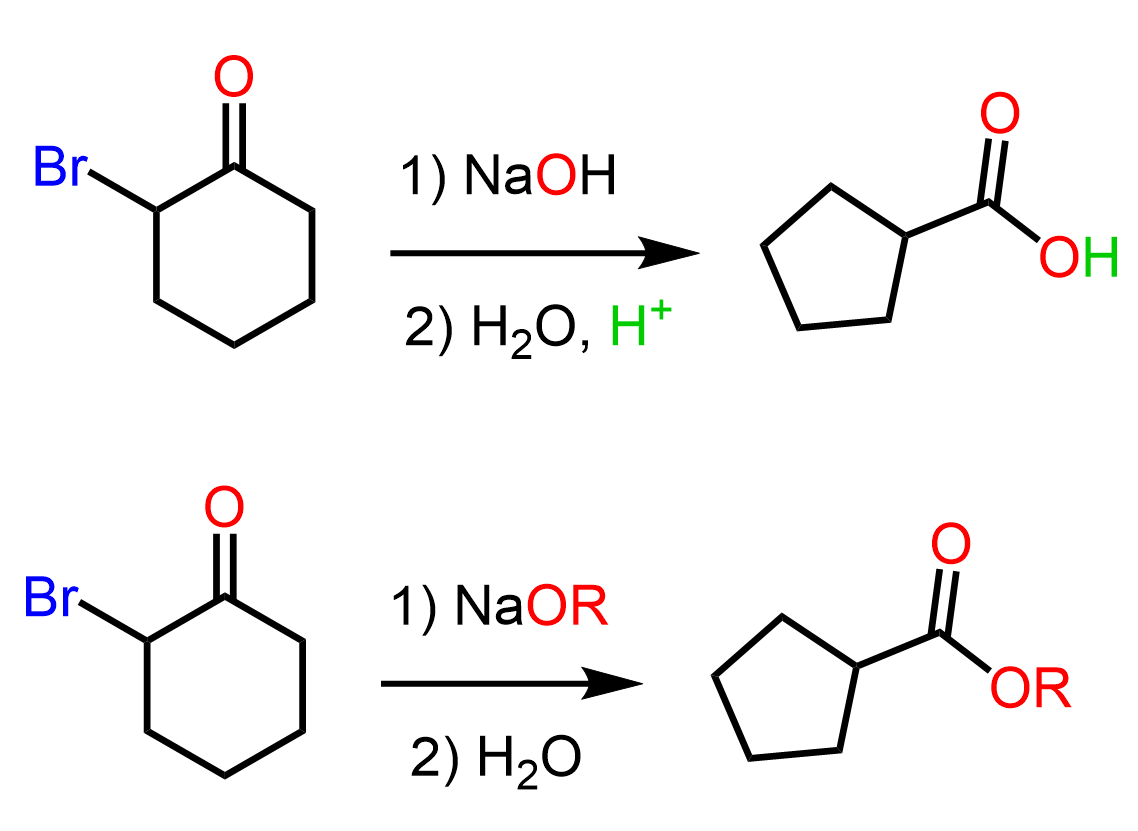
We know that the α position of the carbonyl group is significantly more acidic (pKa ~ 19) than other protons so when treated with a sodium hydroxide or an alkoxide, a deprotonation occurs forming the corresponding enolate ion.
(Yes, if you are wondering how can a hydroxide deprotonate a ketone if the pKa of water is only ~16, it is a good question, and the answer is that the enolate is formed in very little quantitates, and the reaction moves forward as the enolate undergoes a reaction. You can check this article for more details on enolate and aldol reaction).
The enolate then undergoes an internal cyclization forming an highly strained cyclopropenone ring which is opened up by a nucleophilic attack of the hydroxide or alkoxide ion followed by an elimination (check the addition-elimination mechanism):
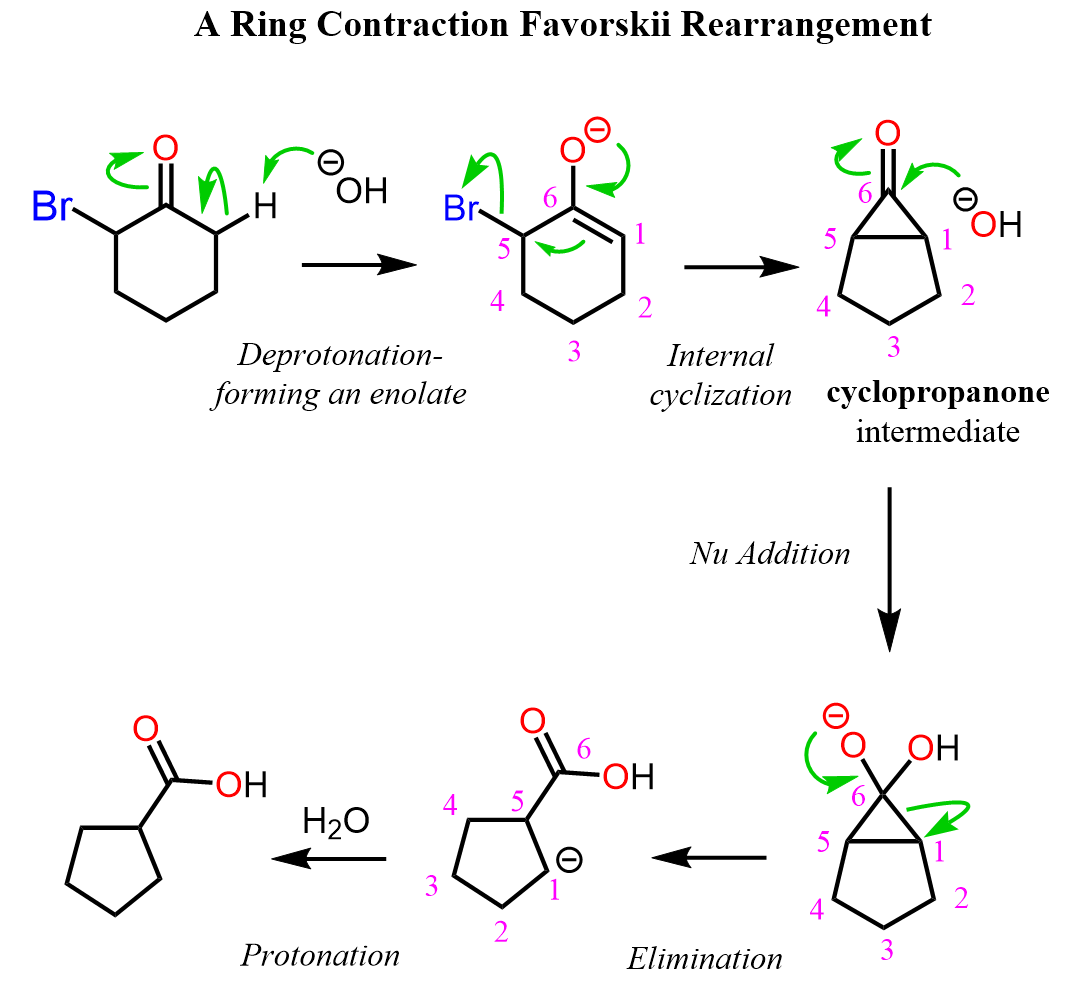
In the last step, the carbon and the carboxylate ion are protonated and a new 5-membered ring is formed.
As mentioned in the beginning, the Favrskii rearrangement is not restricted to ring contraction of cyclic halo ketones. It covers a broad spectrum of substrates and functional groups, but this is not a typical material in undergraduate coursework of organic chemistry (at least in North American textbooks).
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions with Tons of Practice Problems
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz