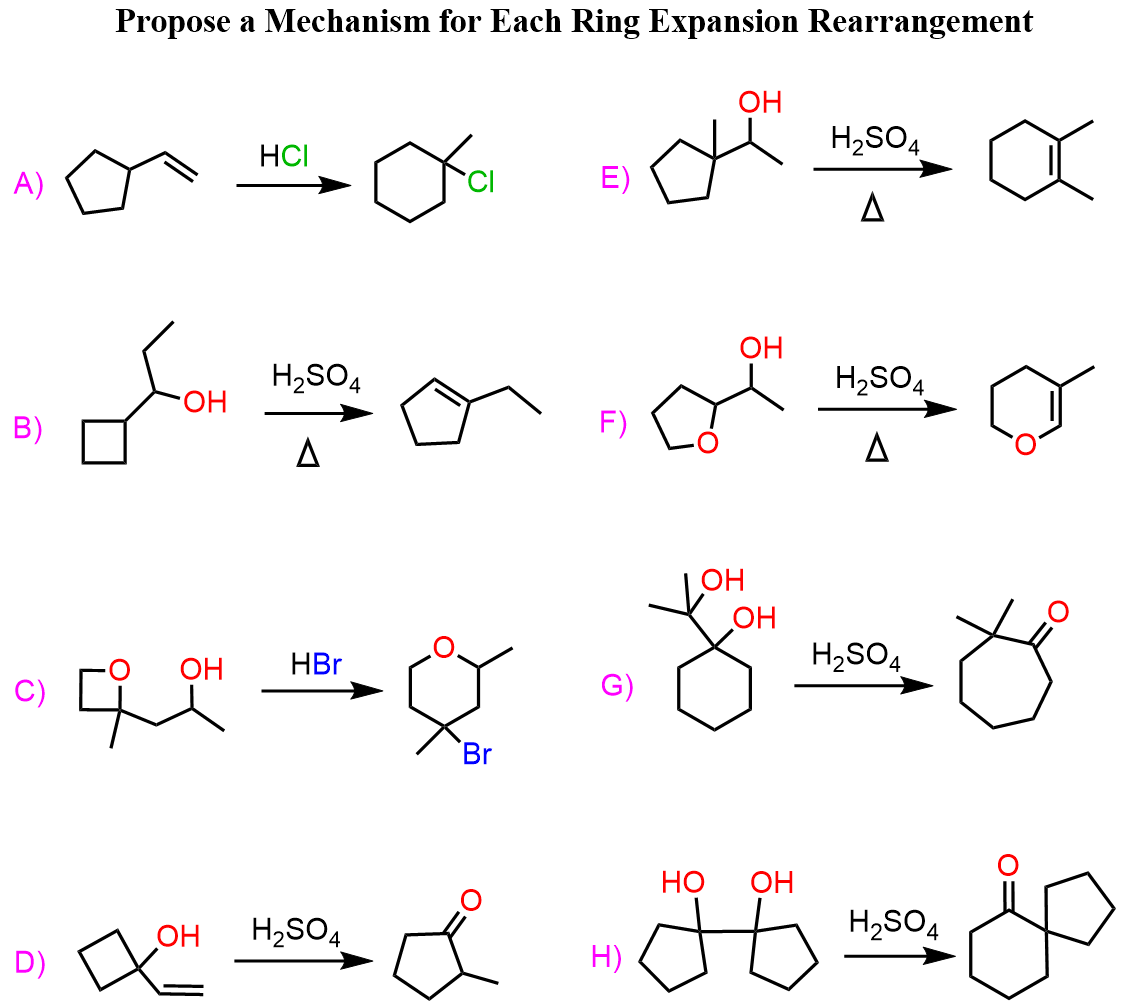
Ring Strain and Ring Expansion
The title of today’s discussion contains two keywords: Ring Expansion and Rearrangement. So, what are those?
- A ring expansion is the transformation of a smaller ring to a larger ring.
- A rearrangement is a change in the connectivity of atoms in the molecule.
Let’s understand the reasons for ring expansion and rearrangements. Ring expansions occur because of the difference in the stability of two cyclic structures. Recall the ring strain, which is the instability caused by angle strain and torsional interaction:

The image below shows the ring strain values of cycloalkanes. The higher in energy, the less stable the cycloalkane is, thus the tendency for ring expansion.
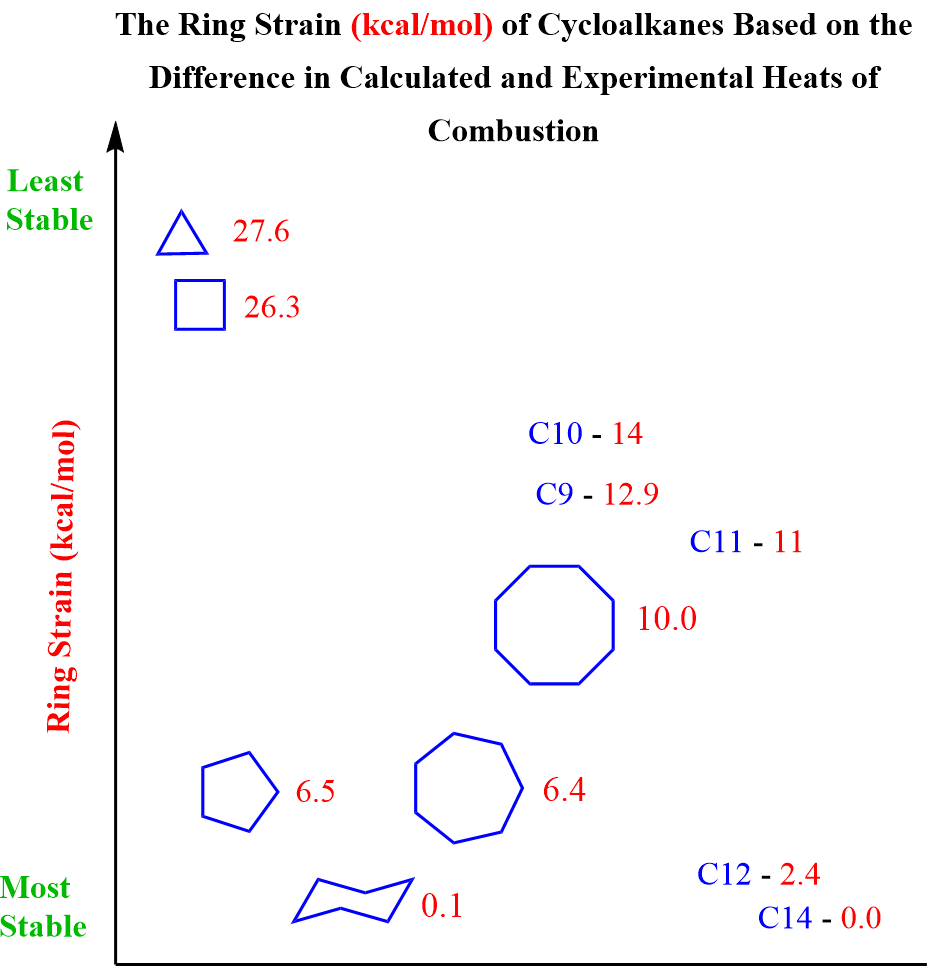
For example, cyclopentane is more stable than cyclobutane; therefore, when possible, a cyclobutane will undergo a ring expansion rearrangement to form a cyclopentane. Although cyclopropane is the least stable among the rings, it does not follow the general trend of undergoing ring expansion rearrangement via carbocation intermediate.
Rearrangements
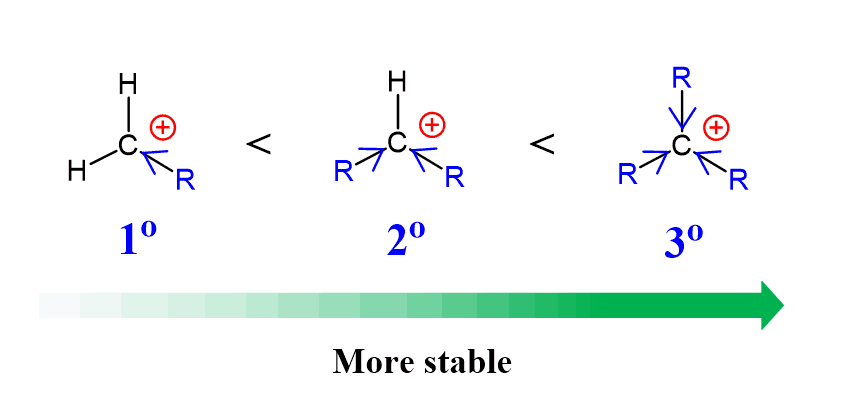
Rearrangements in undergraduate organic chemistry are mostly referred to as carbocation rearrangements. These occur when a less substituted carbocation “finds a way”, such as a methyl or hydride shift, to transform into a more stable carbocation. Recall that the stability of carbocations increases with the number of alkyl groups connected to the positively charged carbon atom:
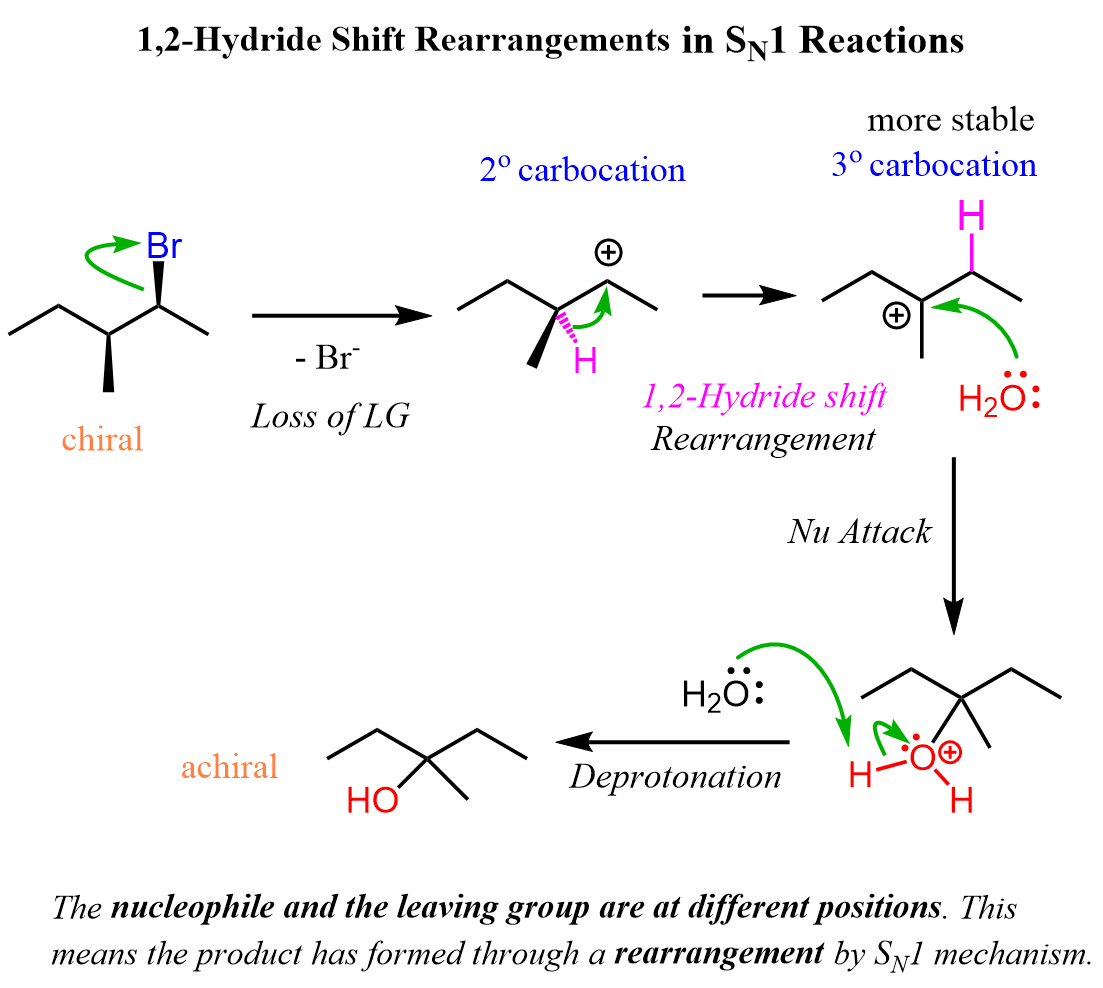
Let’s see an example of carbocation rearrangement in an SN1 reaction. The hydrolysis of the following chiral alkyl halide forms an achiral alcohol because of a 1,2-hydride shift rearrangement of the carbocation intermediate:
Here is an example of a 1,2-methyl shift rearrangement in a hydrohalogenation reaction of an alkene:
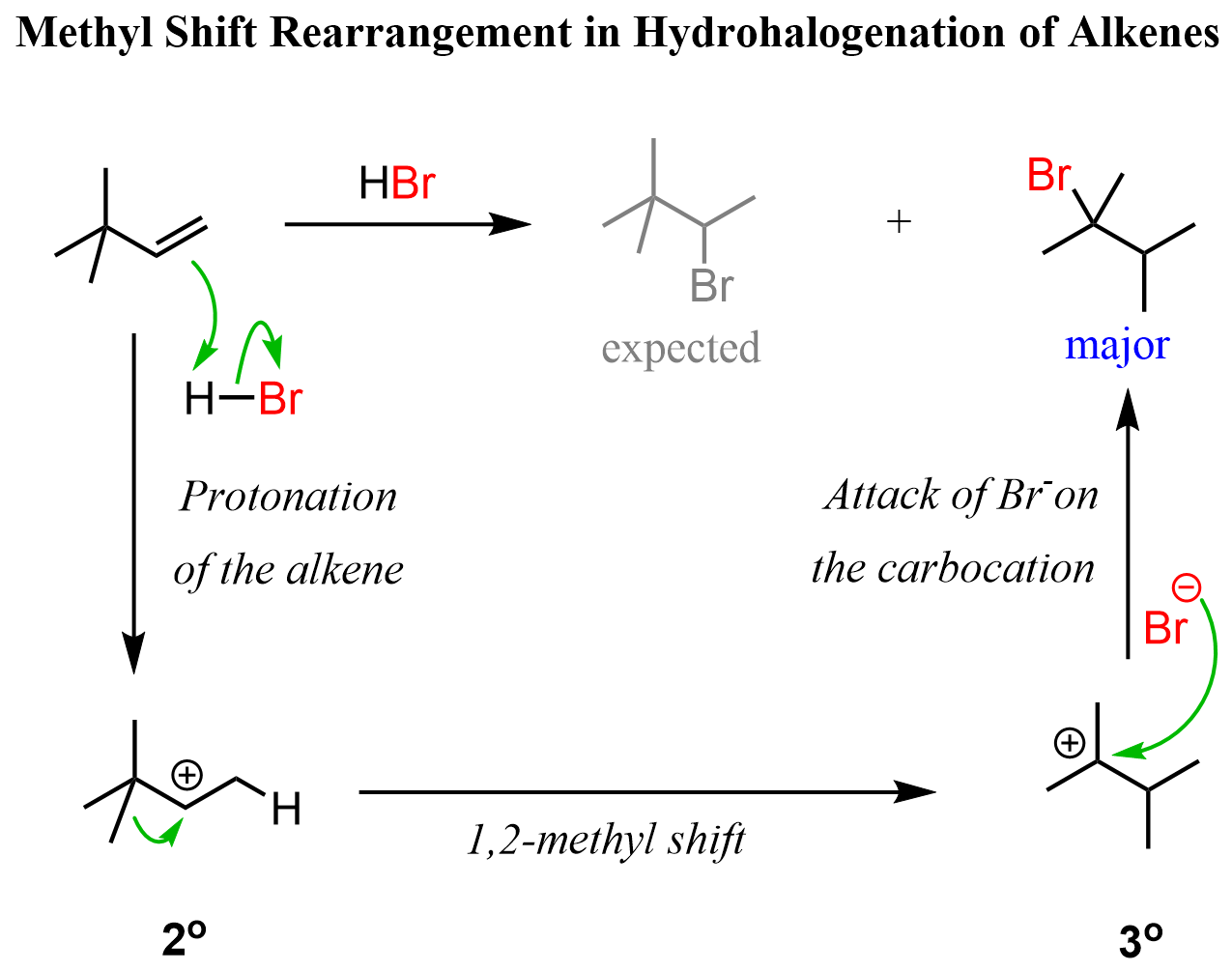
In both cases, the secondary carbocation is converted into a more stable tertiary carbocation. Like the 1,2-methyl shift, ring expansion rearrangement is a type of alkyl shift rearrangement.
Ring Expansion Rearrangement
We have discussed so far that rings and carbocations (essentially like anything else) have the tendency to become more stable, i.e., go into a lower energy level. So, if we combine an unstable ring with an unstable carbocation, we are going to have a ring expansion rearrangement.
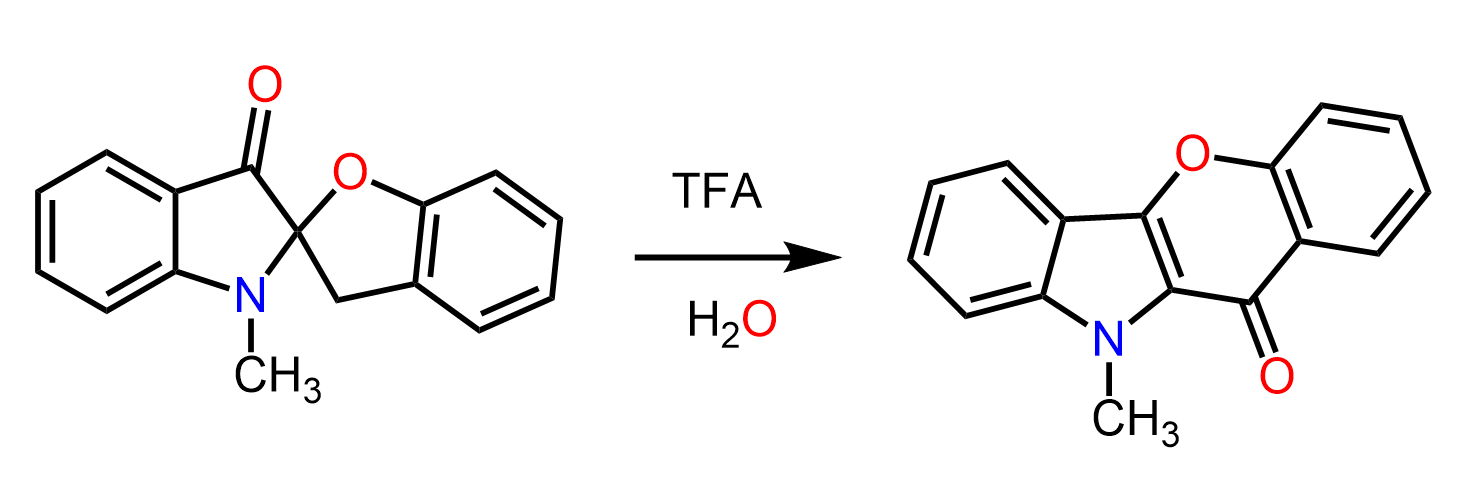
For example, the hydrolysis of the following alkyl halide gives an alcohol where the cyclobutane ring is converted into a cyclopentane:
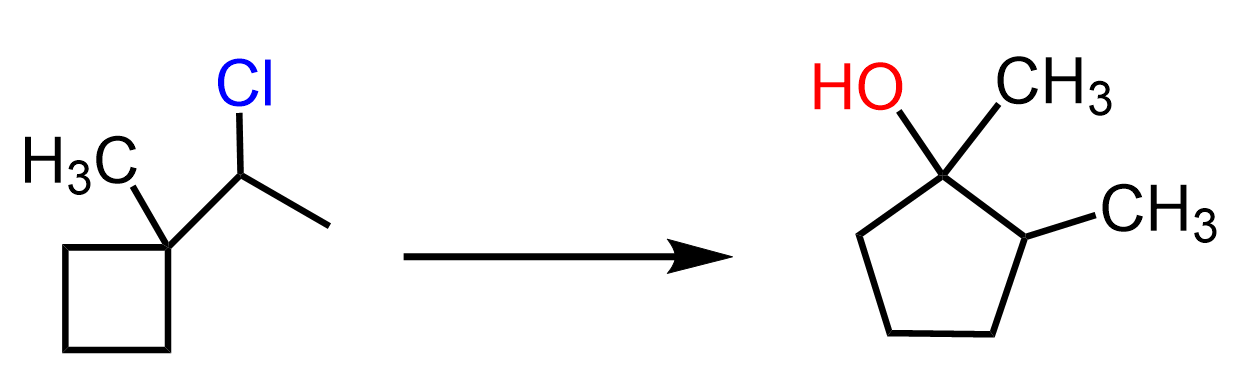
Let’s see how this happens. We have an alkyl halide mixed with water, so we predict that it can undergo an SN1 or E1 reaction. Remember, water is a poor nucleophile and a weak base, and those can only (except for the exceptions, of course) perform SN1 or E1 reactions. The E1 pathway is favored when the reaction is heated, so we know that this must be an SN1 reaction. If this conclusion is confusing for you, check the article on the most common exceptions in SN1 and SN2 reactions.
Like any other SN1 reaction, this as well starts with the loss of the leaving group, and a secondary carbocation is formed. This carbocation is transformed into a more stable tertiary carbocation via a type of alkyl shift called a ring expansion rearrangement:
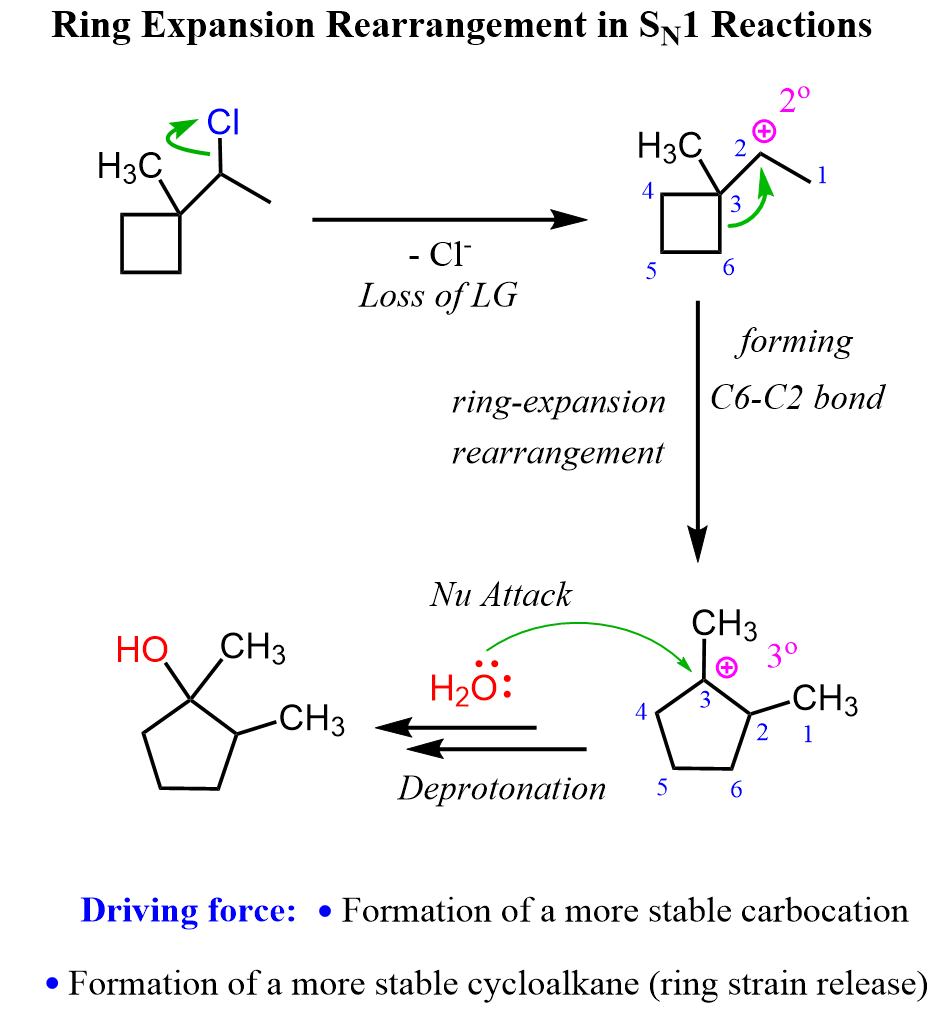
The alkyl is the carbon 6, which forms a new bond with carbon 2 at the same time breaking the bond with carbon 3. As a result, a five-membered ring is formed, which is more stable than cyclobutane. So, there are two driving forces here: increasing the stability of the carbocation and the stability of the ring. Once the tertiary cyclopentyl carbocation is formed, it is attacked by water, forming the final product alcohol.
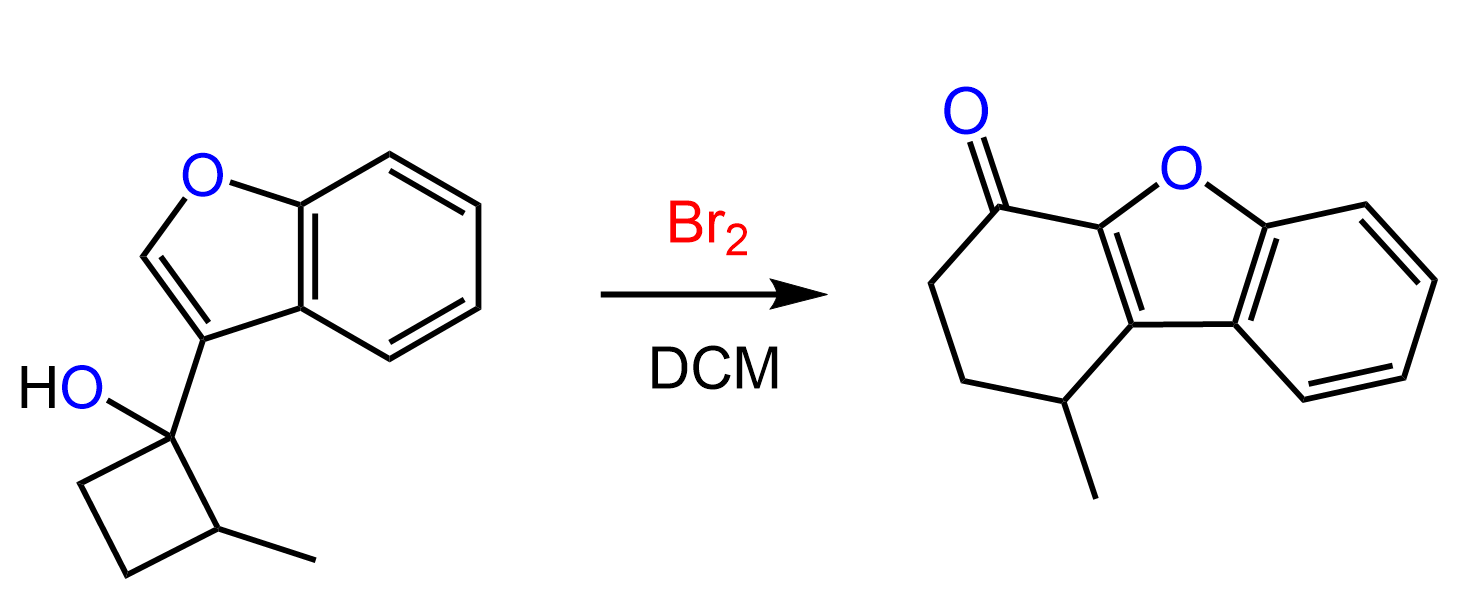
Let’s consider another ring expansion reaction where a cyclopentane derivative is converted into a cyclohexane:
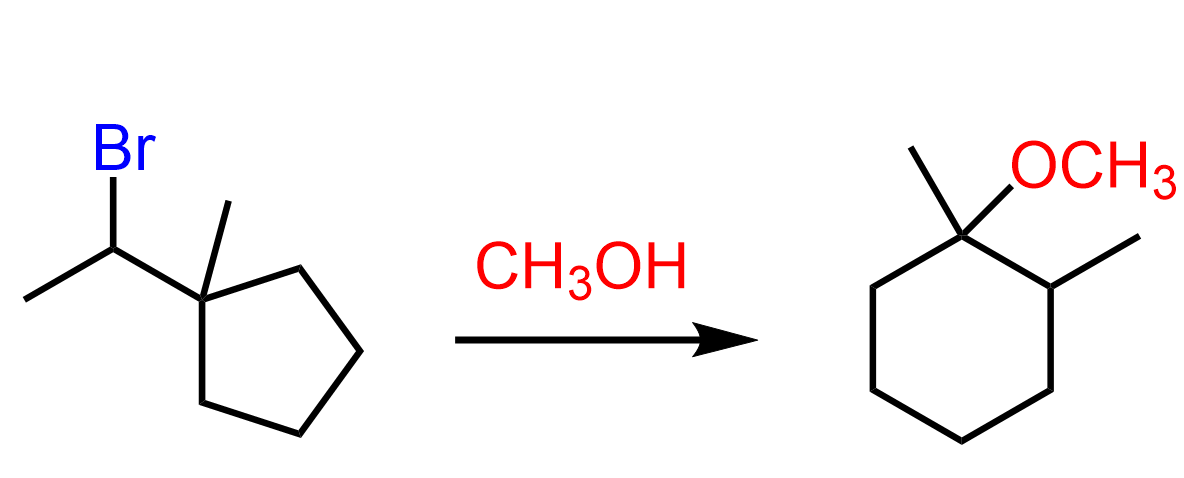
By simply comparing the structures of the alkyl halide and the product, it should be obvious that it is a ring-expansion rearrangement. Take a few minutes to draw a reasonable mechanism for this reaction before looking at what we have below:
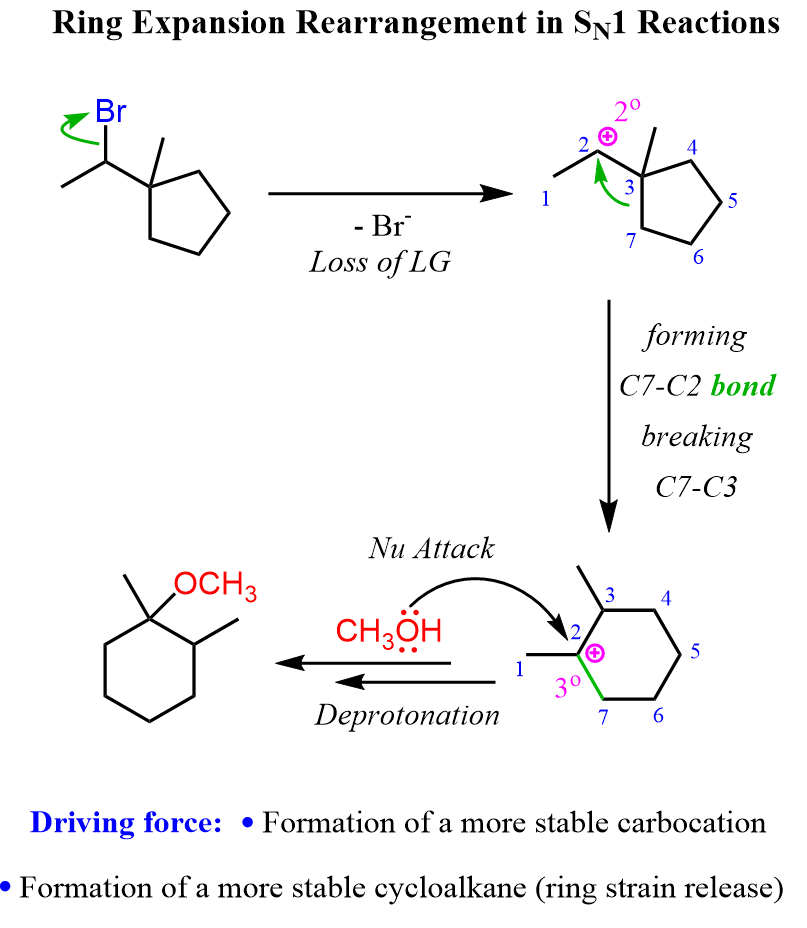
Let’s also consider an example of expansion rearrangement in an elimination mechanism. The following alcohol with a spiro bicyclic structure is transformed into a fused structure of “two” six-membered rings:
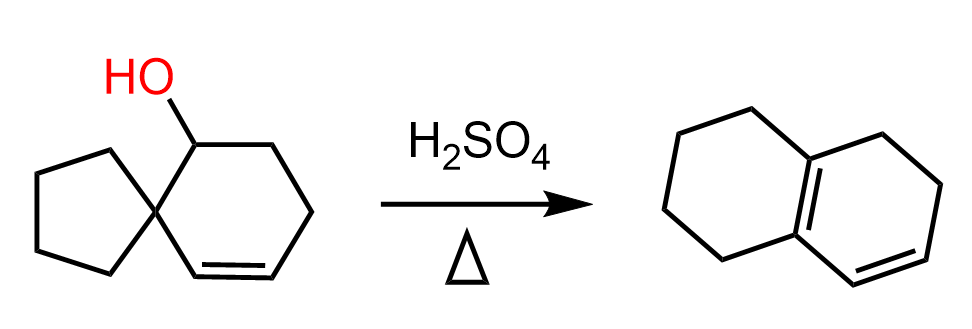
How can we approach this problem? You need to identify the functional groups: we have an alcohol and an alkene reacting with an acid. So, either the OH or the double bond is going to be protonated. Generally, the OH will be protonated first because the protonation of the double bond means breaking it, which is energetically unfavorable. Next, we recall that alcohols undergo E1 elimination when treated with strong acids, and the process goes via the formation of a carbocation. So, let’s go ahead and do that:
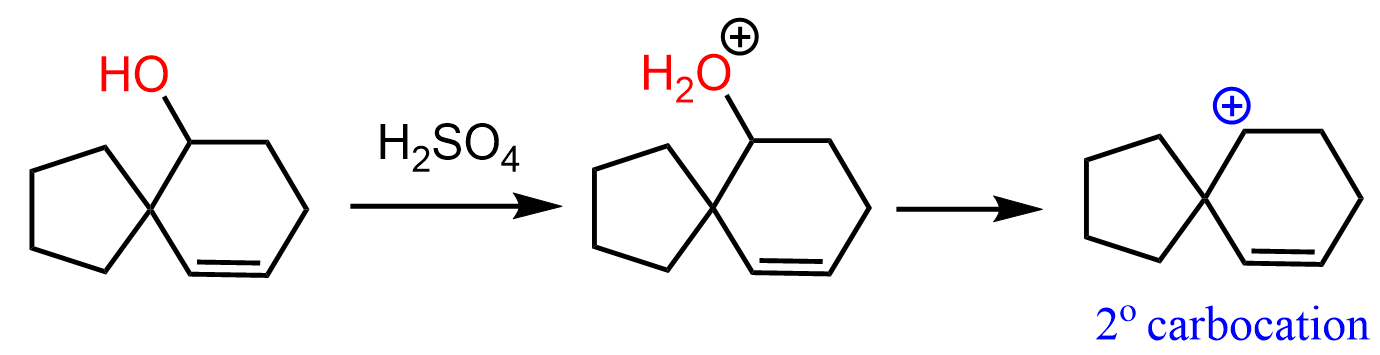
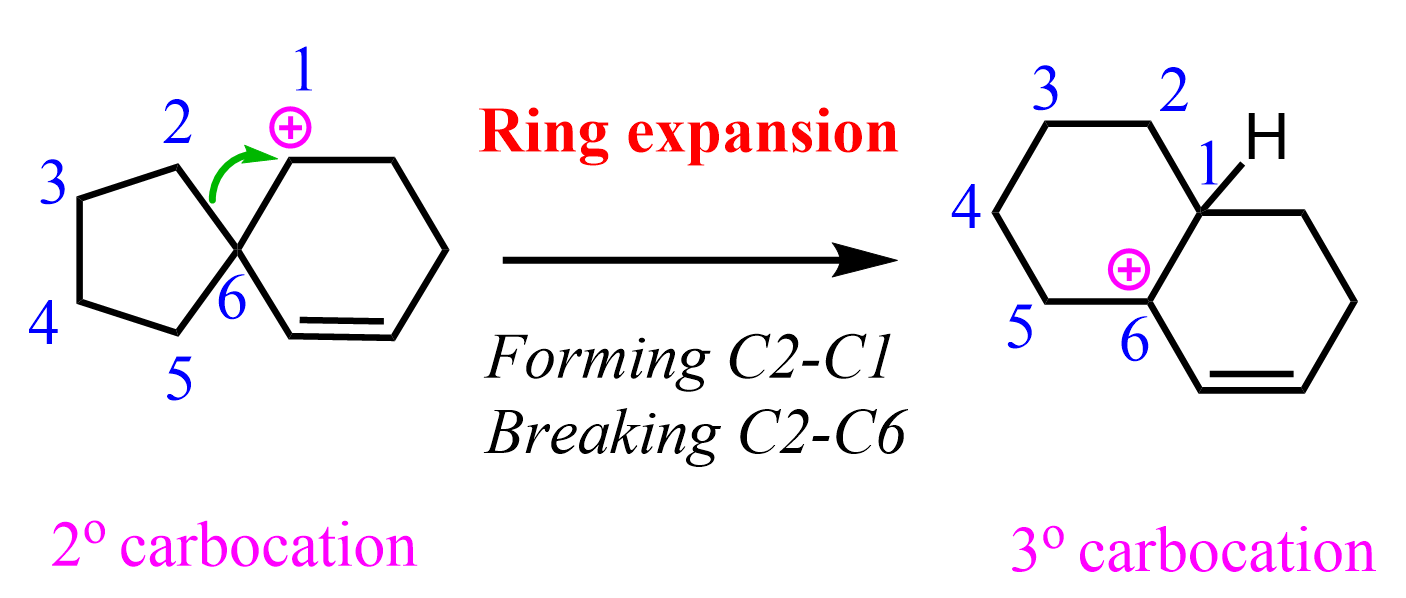
At this point, we need to find a way to convert this secondary carbocation into a more stable one. There are no adjacent hydrogens moving, which would convert this into a tertiary carbocation. Besides, that wouldn’t change the carbon connectivity of the molecule.
Instead, we are breaking the C-C bond on the spiro center, forming a tertiary carbocation and a fused bicyclic system:
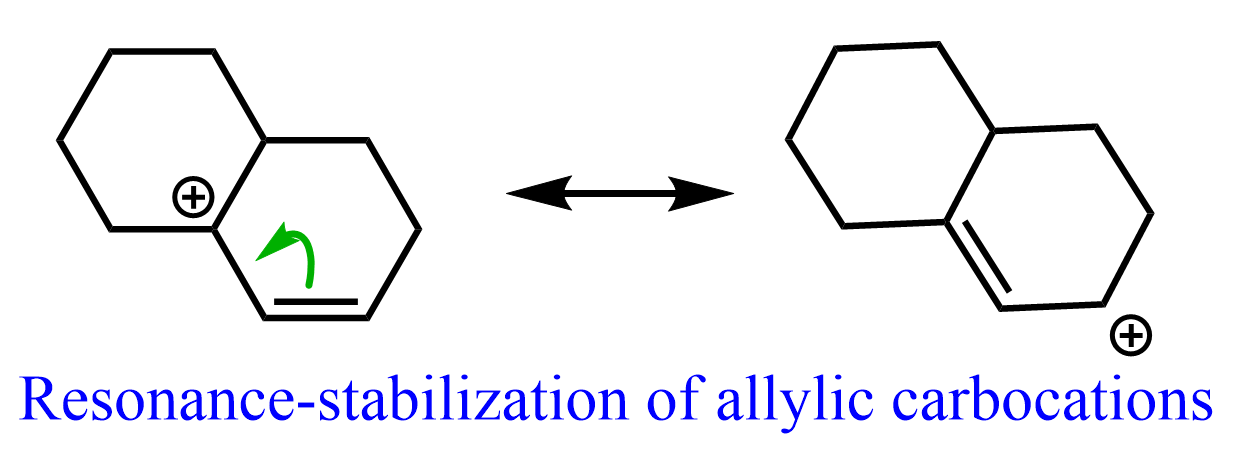
Notice that aside from being a tertiary carbocation, it is also resonance-stabilized thanks to the adjacent double bond. So, it is an allylic carbocation which is generally very stable:
In the last step, deprotonation occurs, forming a conjugated diene, which again is an additional factor driving the reaction forward. Let’s put all these steps together to show the complete mechanism of this ring expansion rearrangement:
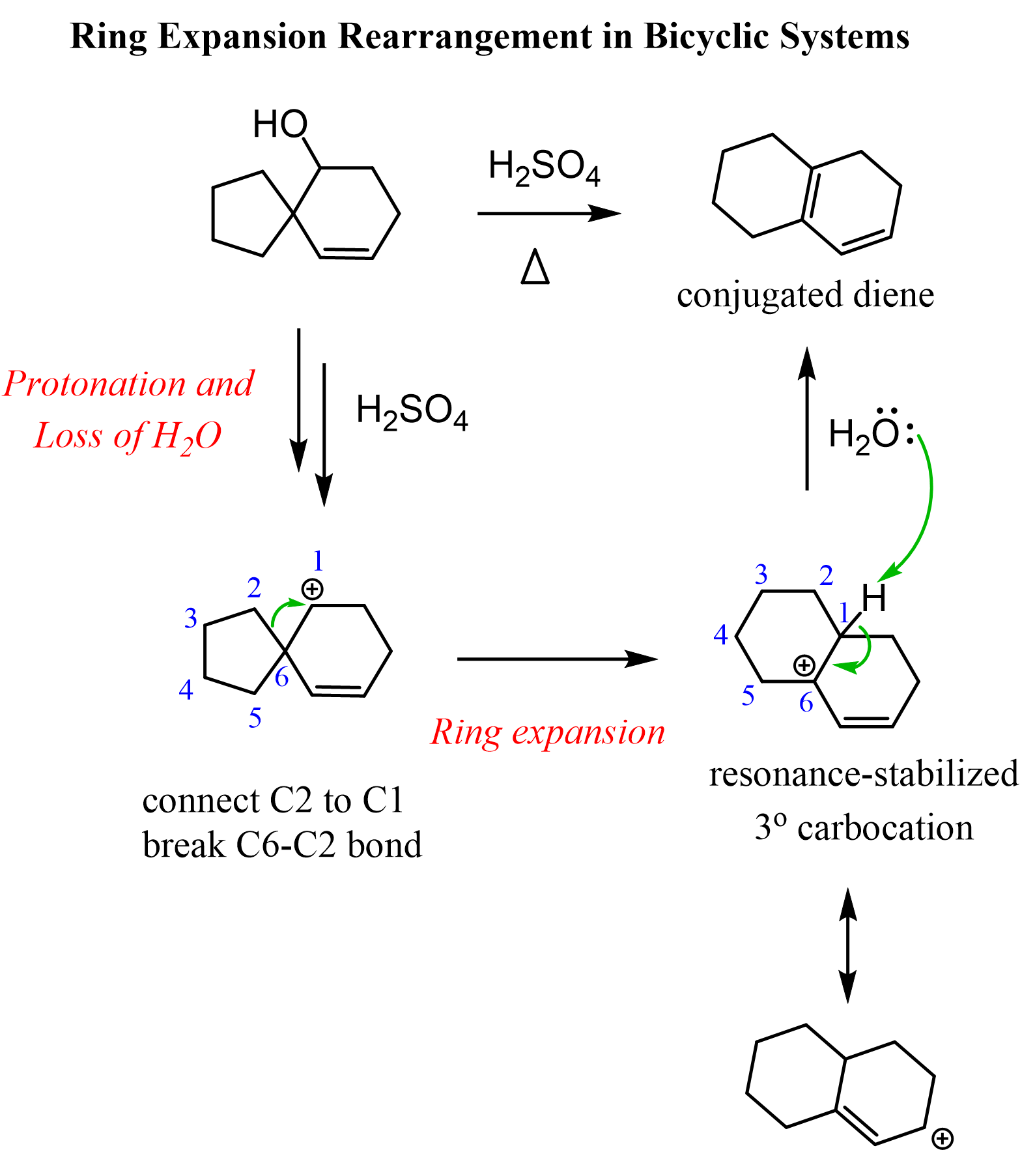
Summary of Ring Expansion Rearrangements
Ring expansion rearrangements happen when two key forces align: the relief of ring strain and the stabilization of carbocations. Smaller, strained rings like cyclobutane tend to expand into more stable five- or six-membered rings when carbocation intermediates form. Always watch out for rearrangements in SN1 and E1 reactions, as they proceed via carbocation intermediates. Formation of a carbocation on small rings, such as cyclopropane (more advanced, less common in undergraduate organic chemistry) and cyclobutane, is a clear indication that a ring-expansion rearrangement is the likely reaction path.