This article is written to address the topic that starts causing lots of confusion when the explanation deviates from the “in SN2, a wedge becomes a dash, and a dash becomes a wedge” statement. So, let’s start with this statement then. What does it mean when they say in SN2, a wedge becomes a dash, and a dash becomes a wedge?
A secondary alkyl halide is chiral at the carbon bearing the halogen; this halogen must be shown by a wedge or a dashed line. For example, in (S)-2-bromobutane, the Br must be shown with a wedge line when drawn in this orientation (remember wedge and dash is relative and it depends on the direction we are looking at):
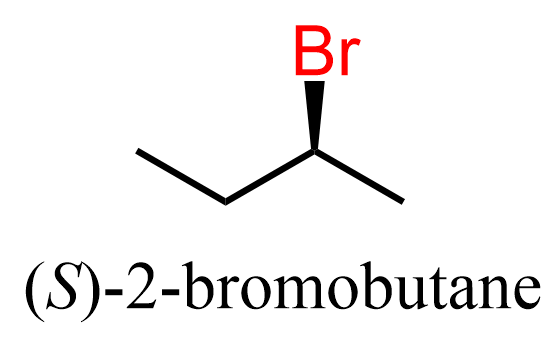
Now, when this molecule is reacted with a good nucleophile such as cyanide ion, the CN replaces the Br, but it is now shown with a dashed line:
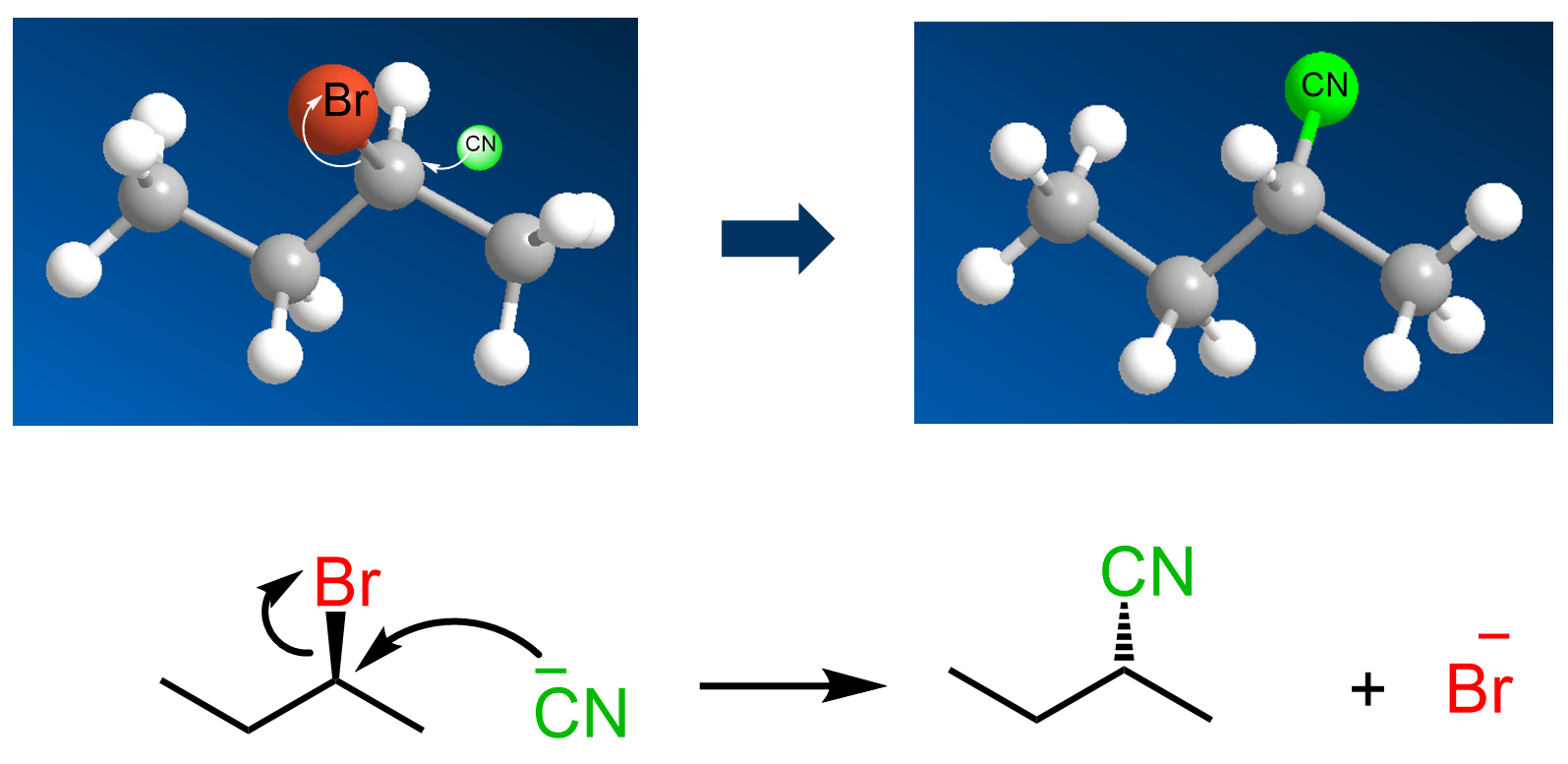
This is because of the mechanism of the SN2 reaction: The nucleophile attacks from the opposite side of the leaving group (backside attack).
So, when the leaving group is wedge pointing towards us, the nucleophile attacks from behind the plane, thus appearing in the product as a dash. If the leaving group is dash, then the nucleophile attacks from our side and appears as a wedge in the product:
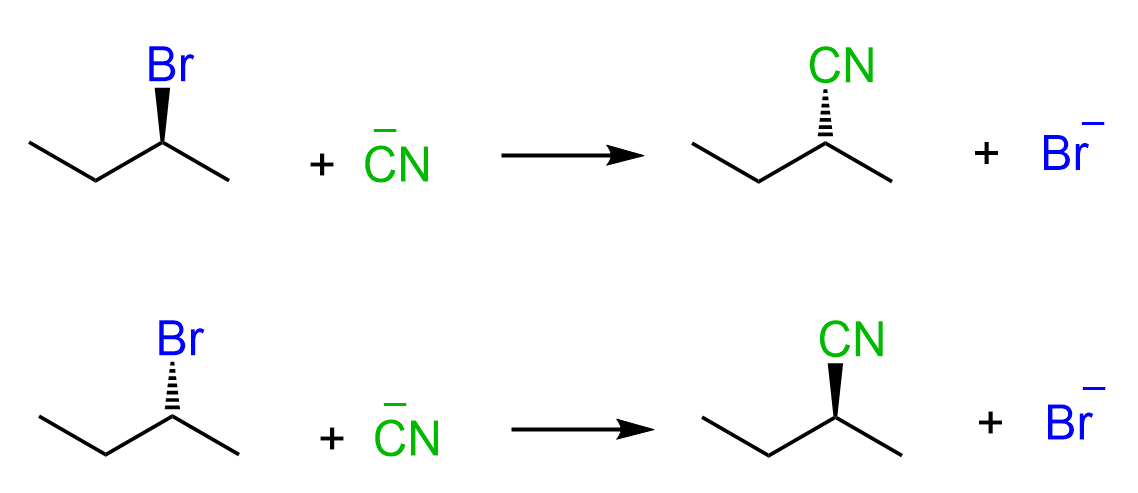
This pattern indicates that the SN2 reaction is stereospecific – the stereochemistry of the product is dependent on the stereochemistry of the starting material.
This feature of the SN2 mechanism is explained by the molecular orbitals (MO theory) of the nucleophile and the electrophile.
According to this theory, the lone pair(s) of the nucleophile is located in the highest occupied molecular orbital (HOMO) and they flow to the lowest unoccupied molecular orbital (LUMO) of the electrophile. The LUMO is the anti-bonding orbital of the C-Halogen σ bond (σ*).
It is the lowest in energy among all the empty orbitals, and therefore, the lone pair of the nucleophile is directed there:
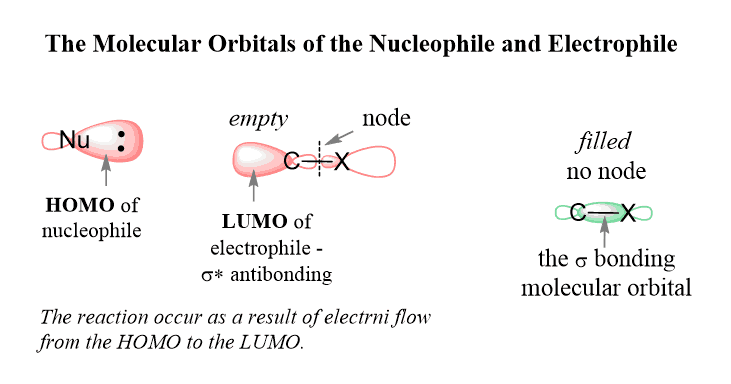
The geometry of the orbitals is such that the antibonding orbital of the C-Br bond is right across the bonding orbital so the only way of occupying this orbital by the lone pairs of the nucleophile is when it attacks at 180o to the leaving group (back-side attack):

More on Wedge and Dash in SN2 Reactions
The wedge and dash notation is handy for a quick guide; however, you need to keep in mind that it is not an ultimate indicator of the reaction mechanism or the configuration of an atom. Wedge and dash simply depend on the direction we are looking from:
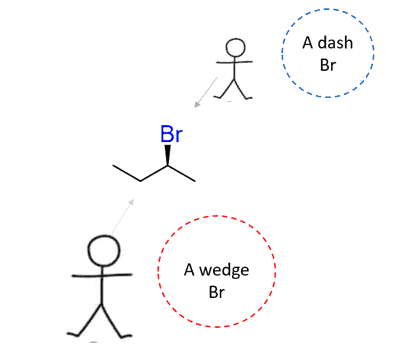
Keep an eye out for tricky questions using the wedge and dash notation.
For example, does the following reaction go by an SN2 or SN1 mechanism?
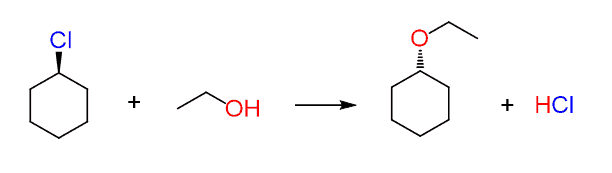
The thing here is that you may look at it and think that since the wedge leaving group is changed into a dash nucleophile, then it must be SN2!?
You should notice, however, that the carbon connected to the leaving group is not chiral since the molecule has a plane of symmetry and the dash line becomes wedge by simply flipping the molecule 180o:
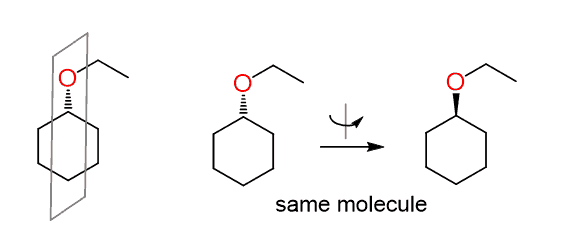
Therefore, sometimes we cannot determine if the reaction is SN2 simply by looking at the wedge and dash lines. In fact, this is a reaction of a secondary alkyl halide with a weak nucleophile and is likely to go by an SN1 mechanism.
Since there is no chirality center, the wedge and dash lines should be normally replaced with plain lines.

The take-home from this example is not to decide on the mechanism by only comparing the wedge and dash notation of the leaving group and the nucleophile.
SN2 Reactions of Achiral Substrates
Let’s also consider what happens if, instead of the alcohol, its conjugate base alkoxide ion is reacted with chlorocyclihexane. Remember that alkoxide ions are good nucleophiles and strong bases; therefore, we are going to decide between SN2 and E2 mechanisms. In most cases, the predominant path of reacting secondary alkyl halides with basic good nucleophiles such as alkoxides is the E2 mechanism.
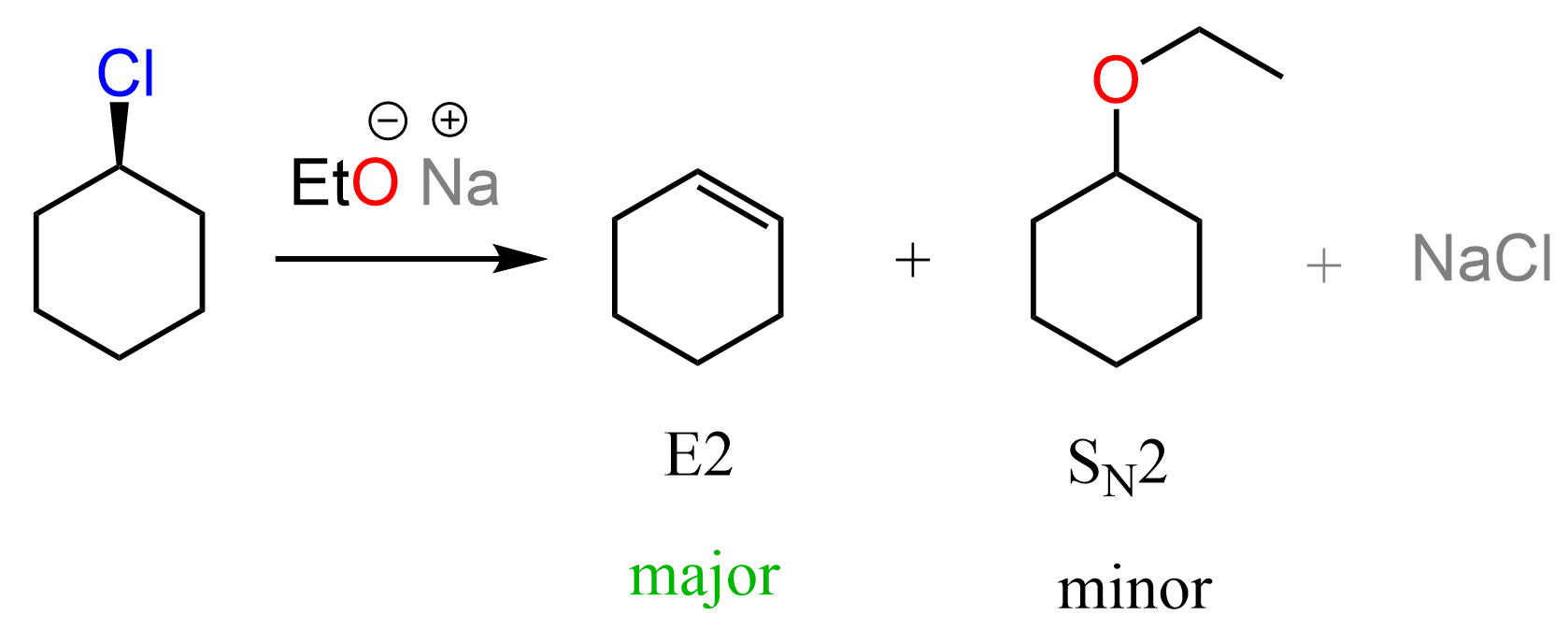
However, the SN2 product is also often observed, and for the purpose of today’s discussion, we will focus on that. You can read more on deciding between SN1, SN2, E1, and E2 mechanisms here.
The product is the same ether as we saw in the SN1 reaction with the alcohol. However, it follows an SN2 mechanism; however, the wedge and dash notations are still sort of irrelevant because the substrate is achiral. Now, why do we say “sort of” irrelevant?
It is important to mention that the mechanism is SN2, which means the nucleophile attacks from the opposite side of the leaving, and thus their “physical positions” in the transition state are different. However, it has no significance in terms of the wedge and dash and even R and S notation because both the substrate and the product are achiral (not chiral).
We can perhaps see this better by drawing the cyclohexane in a chair conformation:
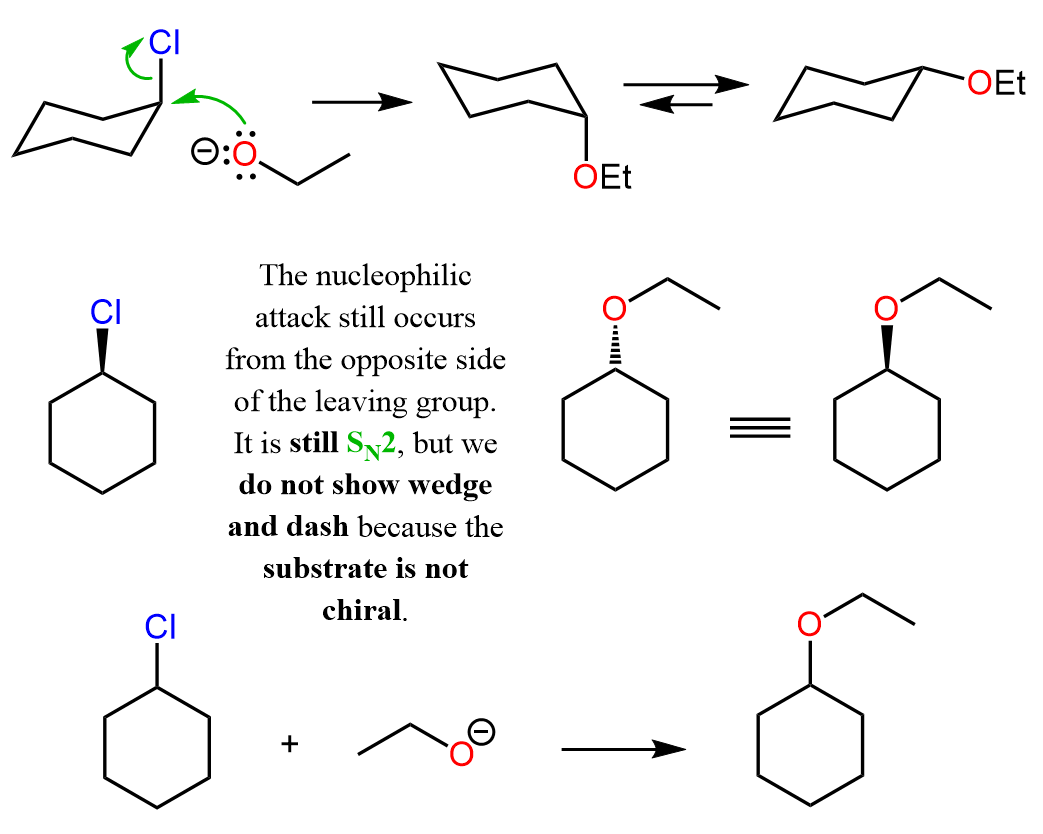
Let’s also consider a similar reaction where the substrate and the product are achiral, but the wedge and dash notation does matter:

The carbon connected to the leaving group is, again, not chiral, and the molecule has a plane of symmetry. However, in this case, we do need to show the wedge and dash lines, and it is not to make it tricky.
The difference with the previous reaction is that there is a methyl group here pointing towards us, and depending on whether the nucleophile appears as a wedge or dash, we get two different products:
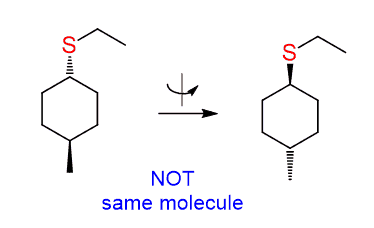
These are cis and trans isomers – a pair of diastereomers and, of course, you can’t do the 180o flip again.
Now, if the reaction goes by an SN2 mechanism, then the nucleophile should appear as a dash – anti to the methyl group since it attacks from the opposite side of the leaving group:
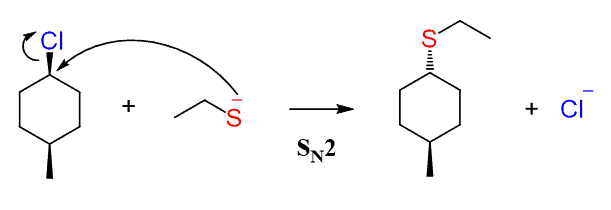
The SN1 reaction would have resulted in a pair of diastereomers since it forms a carbocation intermediate before the nucleophilic attack:
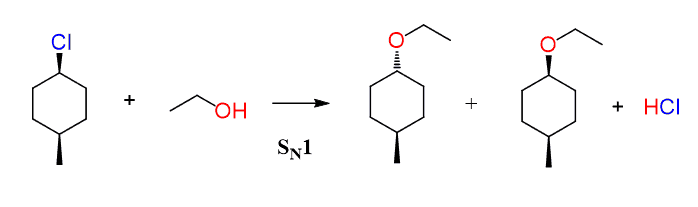
Notice that in any case, the configuration of the methyl group does not change as it is not part of the reaction.
SN2 With Retention of Configuration
As we discussed above, the only sure way of determining whether there was an inversion of configuration (Walden inversion) or not is by comparing the absolute configuration of the starting material and the product (R and S).
But what if you see a reaction when the absolute configuration of the ɑ-carbon stays the same?
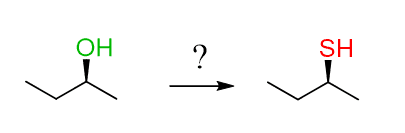
How do you know if this is SN2 or SN1, since we know that the configuration changes from R to S or S to R if it is SN2, and it racemizes in SN1 reactions (check the stereochemistry of SN1 reactions here)?
Well, the SN1 is out since the product is shown as a single enantiomer, meaning that the other enantiomer is not formed.
And the question is, how do you perform an SN2 and keep the configuration the same?
The starting material here is S, and an SN2 reaction would change it to R. However, if we do two SN2 reactions it will go S-R-S:

One approach for achieving this would be converting the OH into a halide, followed by a reaction with sodium sulfide. There are different ways of converting an alcohol into a good leaving group, and you may not be familiar with some of them now:

The method shown above uses the mesylation of alcohols to convert them into a good leaving group, which can then be expelled by the Br– and undergoes another SN2 reaction with the sulfide ion.
This was a three-step synthesis applying double SN2 to retain the absolute configuration, but:
Can an SN2 reaction process occur with retention of configuration?
This is rather an exception that you will likely not need to deal with, but yes, you can have an SN2 reaction where the absolute configuration is not changing.
For example, in the following reaction, the absolute configuration does not change even though it goes through an SN2 mechanism:

The reason for this is that, unlike the leaving group, the nucleophile is not the highest priority on the chiral carbon:

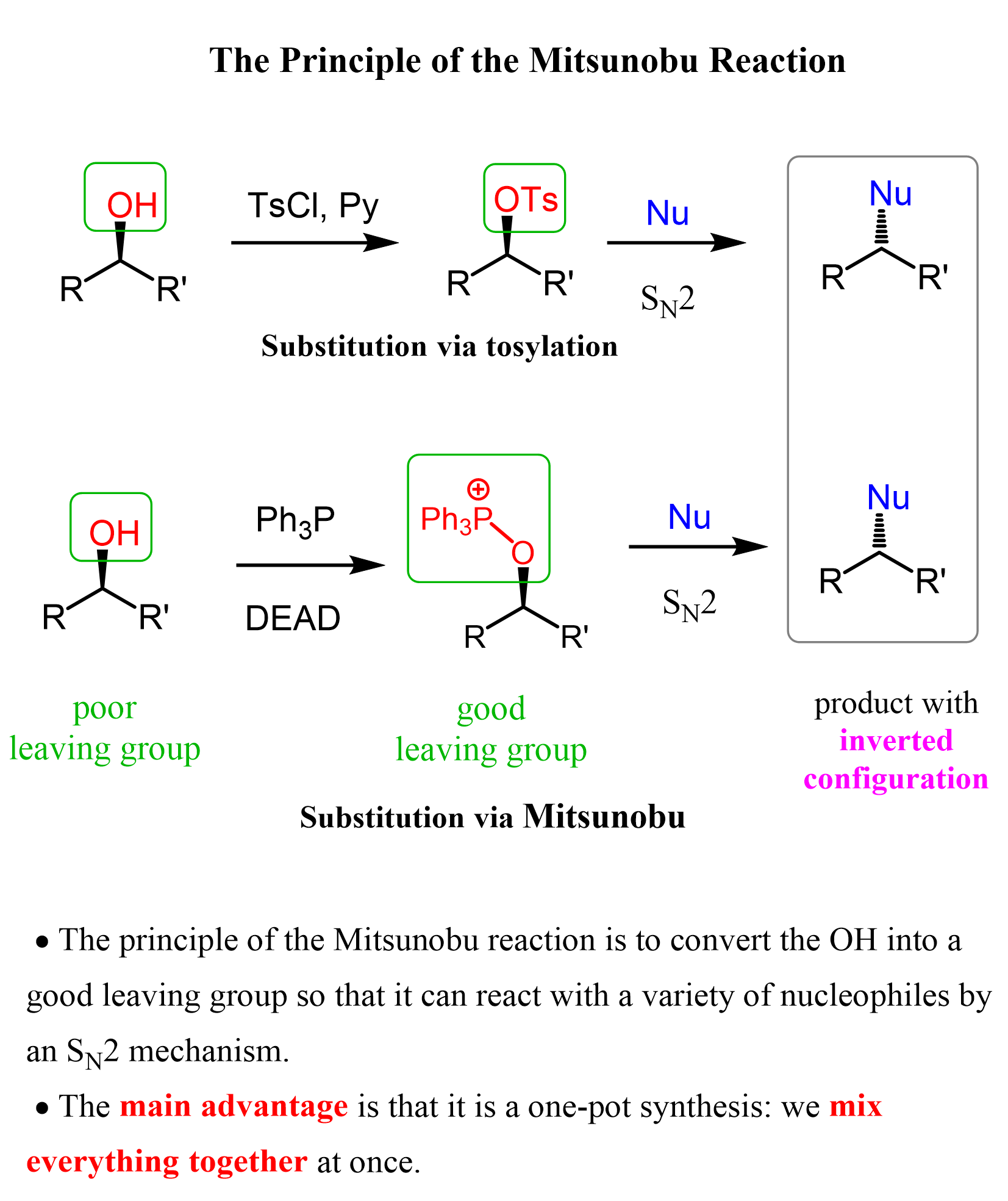
Primary and secondary alcohols can also serve as the electrophile in an SN2 reaction, by converting the OH into a good leaving group such as a mesylate, tosylate, or triflate, or by using the Mitsunobu reaction, which allows this transformation in one-pot synthesis:
There are other tricky situations that you will encounter in SN2 and SN1 reactions, and we have a separate post discussing them, so feel free to check that out as well.
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides

Hi,
I don’t understand, why there is written that the reaction of chlorocyclohexane with ethanol goes by Sn1 mechanism: “In fact, this is a reaction of a secondary alkyl halide with a weak nucleophile and is likely to go by an SN1 mechanism.”, but the figure below shows mechanism according to Sn2: “the nucleophilic attack still occurs from the opposite side of the leaving group. It is still Sn2”.
Could you make it clear to me?
Hello,
The second part was supposed to be the conjugate base of ethanol. Fixed – thanks for bringing that up, and don’t hesitate to ask if you have more questions!