We have seen countless examples of stereoisomers, such as enantiomers and diastereomers, which arise due to different absolute configurations of chiral centers. We learned that chiral centers are only possible when the carbon is connected to four different groups, which presumes only single bonds on that carbon.
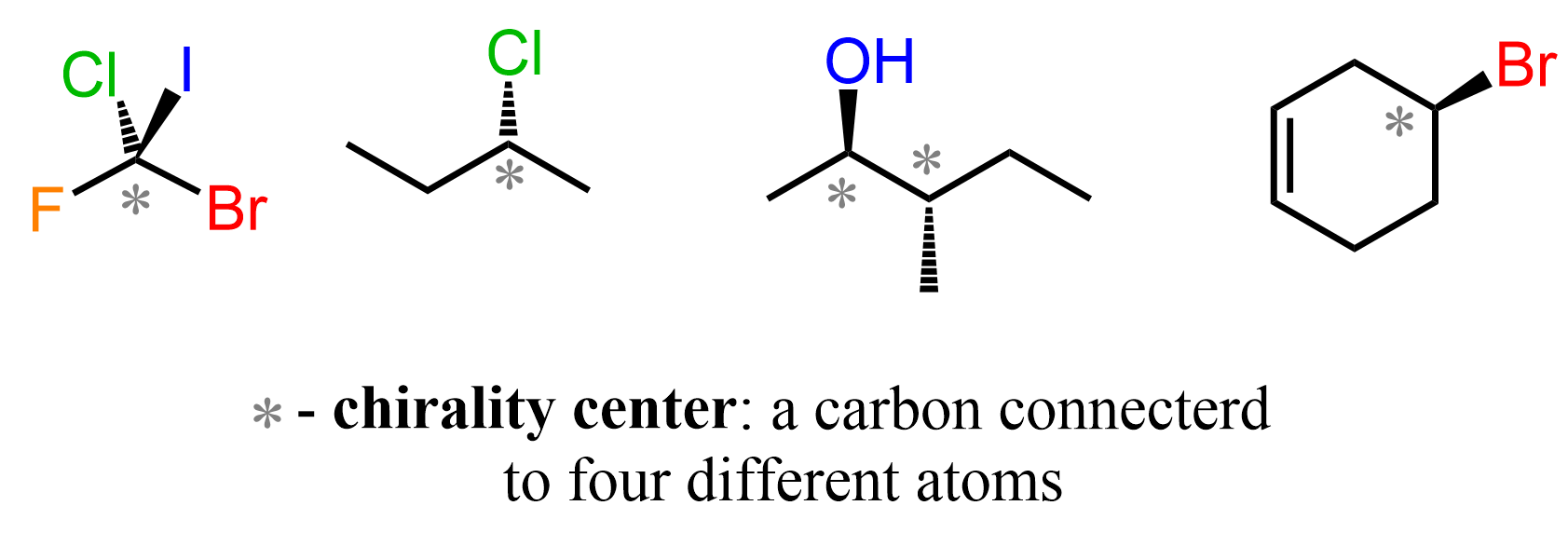
So, what are we talking about when saying “stereochemistry of alkenes”? The stereochemistry of alkenes refers to how atoms or groups are arranged around the carbon-carbon double bond (C=C). Yes, it turns out that having groups connected on different sides of the double bond can give alkenes with different spatial arrangements of atoms. For example, in simple but-2-ene, we can have the hydrogens and methyl groups on the same side, or on opposite sides of the double bond:
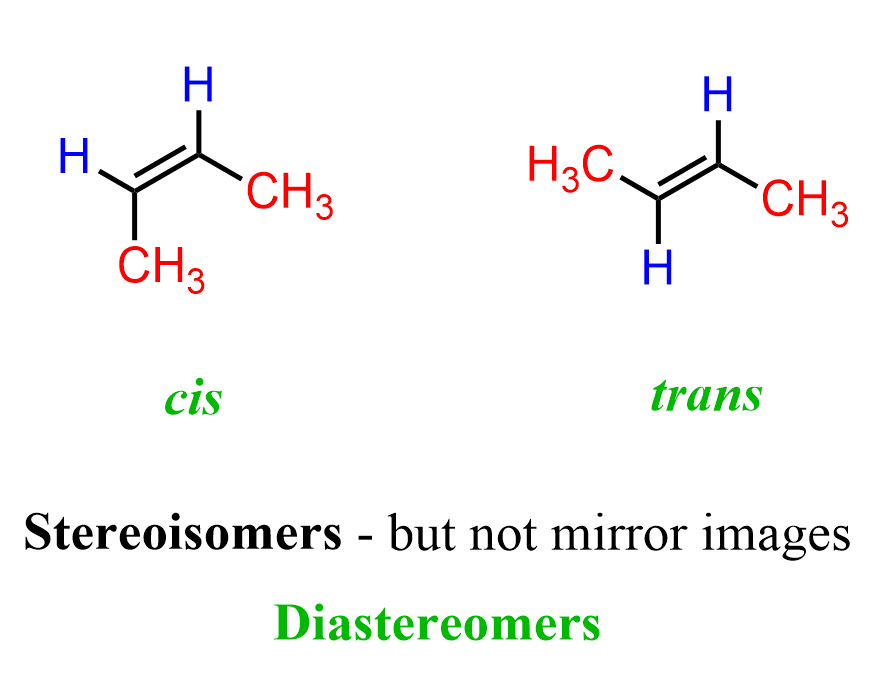
These are stereoisomers because the molecules have the same connectivity of atoms, but different spatial arrangements. We call these cis and trans isomers. In cis isomers, the similar or higher-priority groups are on the same side of the double bond, while in trans isomers, the similar or higher-priority groups are on opposite sides of the double bond, giving the molecule a more linear and extended shape.
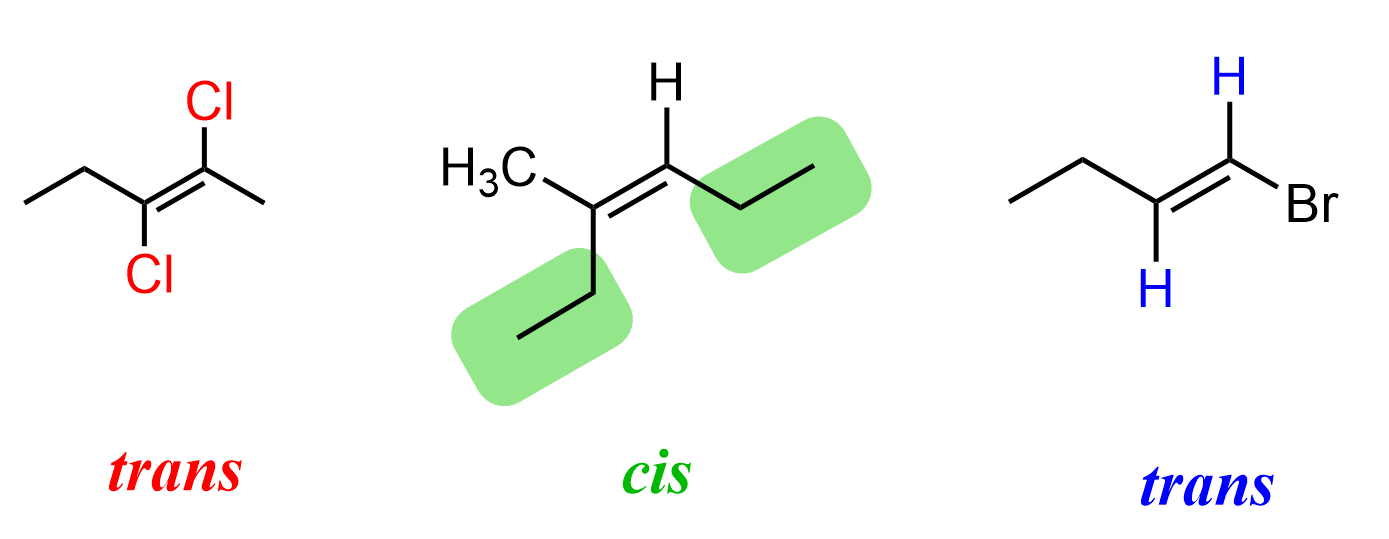
Here are some more examples of cis and trans alkenes:
Restricted Rotation
You may be wondering why we cannot rotate the double bond and change the position of the methyl and hydrogen groups like we did in the case of butane.
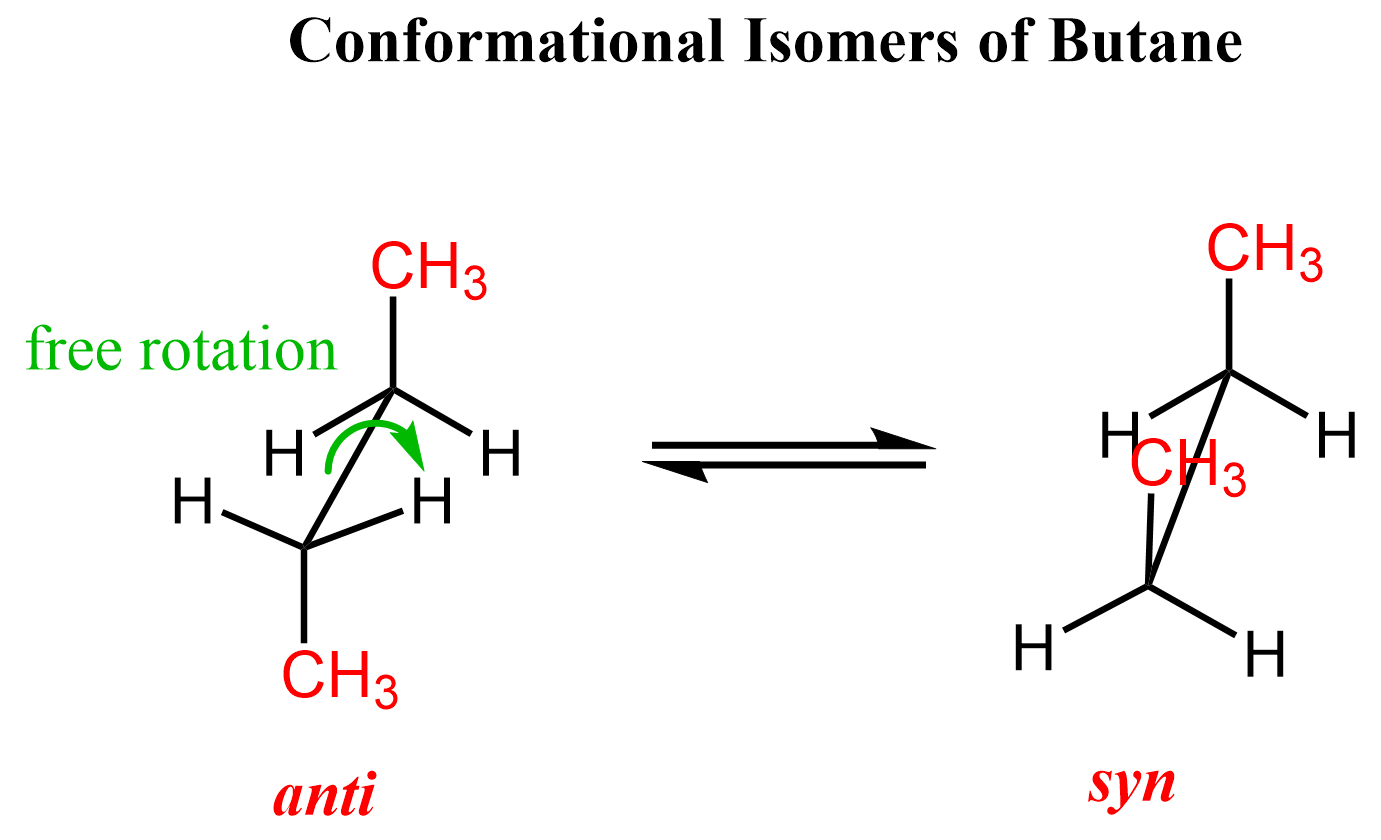
These two are conformational isomers of butane, which are interconverted via free rotation about the central C-C single bond. When the large groups are aligned, at 0o dihedral angle, we have the syn conformation, and when they are at 180o, we have the anti-conformation.
Now, the situation becomes different if the free rotation about single bonds is restricted. Unlike single bonds, which can freely rotate, a double bond restricts rotation because of the π bond. This restricted rotation gives rise to geometric (cis/trans or E/Z) isomerism.
This means that if we have the methyl and hydrogen groups on the same or opposite sides of the double bond, we can’t interchange them anymore:

These two different spatial arrangements of atoms in butene or any other alkene make two distinct molecules called cis and trans isomers.
E and Z Configuration
The cis and trans notation works only if each carbon of the double bond has at least one identical group, because it relies on the simple idea of “same side” versus “opposite side.” We are talking about having the same group on both carbo atoms of the double bond – not on the same carbon because, as we will see, there is no stereoisomerism in this case.
When both carbons of the double bond have different groups, this system becomes ambiguous and cannot uniquely describe the stereochemistry.
To illustrate this limitation, let’s consider two isomeric alkenes having four different groups on the double bond.

In this case, we cannot simply say “cis” or “trans,” because there is no obvious pair of identical groups to compare. Instead, we use the E-Z system, which assigns priority to the substituents on each carbon using the Cahn-Ingold-Prelog rules (R and S configuration).
Determining E and Z Configuration
The E/Z designation clearly specifies the arrangement of the higher-priority groups, avoiding the ambiguity of cis/trans notation.
It is determined based on the priorities of the groups on the double bond:

For example, we have seen that this alkene cannot be classified as cis or trans, but is it E or Z?

Let’s first focus on the left carbon. It has an ethyl group and a Cl connected to it. The Cl has a higher priority because of its atomic number:

On the right carbon, we need to compare a hydrogen with a Br atom. Clearly, the Br has a higher priority:

Finally, determine whether the higher priority group on each carbon is on the same or opposite side of the double bond. Since Cl and Br are pointing up and down, they are on opposite sides, and the alkene has an E configuration:

You may wonder what the Z configuration of this alkene would’ve looked like. Right below:

Once again, you can check this post on E and Z configuration here for more details and examples.
When Cis-Trans and E-Z Don’t Work
There are cases where an alkene cannot have cis-trans or E-Z isomers, specifically when one of the double-bonded carbons is connected to two identical groups. In this situation, there is no difference in priority between the substituents on that carbon, so the molecule cannot exist as distinct geometric stereoisomers. For example, the following two alkenes cannot have cis-trans isomerism because one of the double-bonded carbons bears two identical groups.
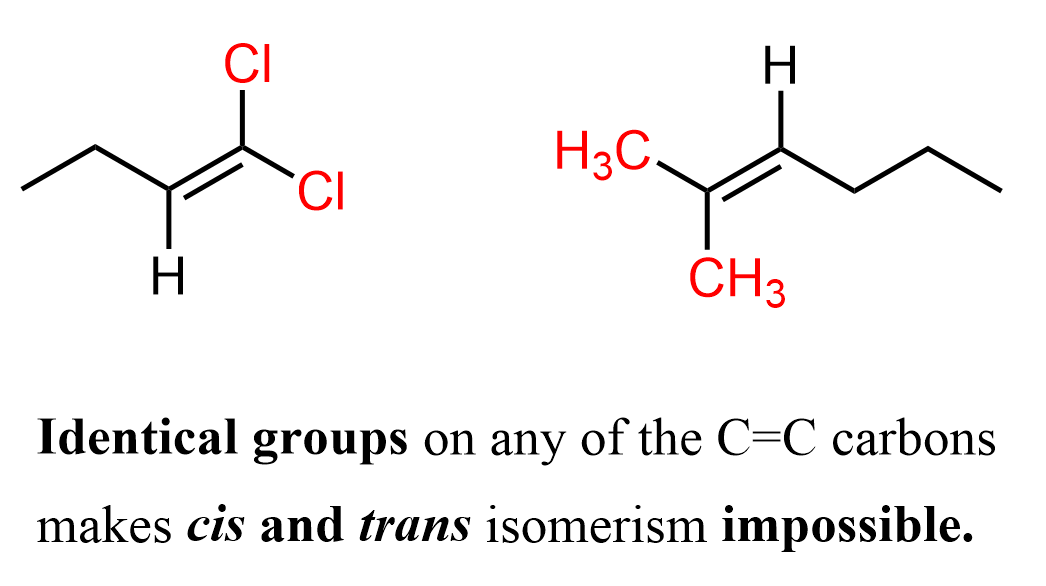
Notice that we are talking about identical atoms or groups on the same carbon atom of the double bond. Cis and trans isomerism is only possible if both carbons in the C=C have two different atoms or groups attached.
Since cis-trans relies on “same side versus opposite side” and E-Z relies on assigning higher-priority groups, both systems fail when the two groups on a carbon are identical. As a result, the alkene is non-stereogenic at that double bond and exists as a single, unique structure.
Stereogenic Center vs Chiral Center
We said chiral centers are stereocenters, and they have four different groups connected to them. Now, we are saying cis and trans isomers are also stereoisomers, but the carbons of the double bond have only three groups attached. So, what is going on here?
The key is that stereogenic centers are not limited to tetrahedral carbons. Any atom or structural feature that gives rise to non-superimposable stereoisomers can be a stereogenic center. In the case of alkenes, the double bond restricts rotation, so the two carbons of the C=C act as stereogenic centers, giving rise to geometric isomers (cis/trans or E/Z) even though each carbon is bonded to only three groups.
For example, both sets of pairs shown below are stereoisomers because they contain carbon(s) with a stereocenter. One of them, however, is also a chiral center, as it is connected to four different groups.
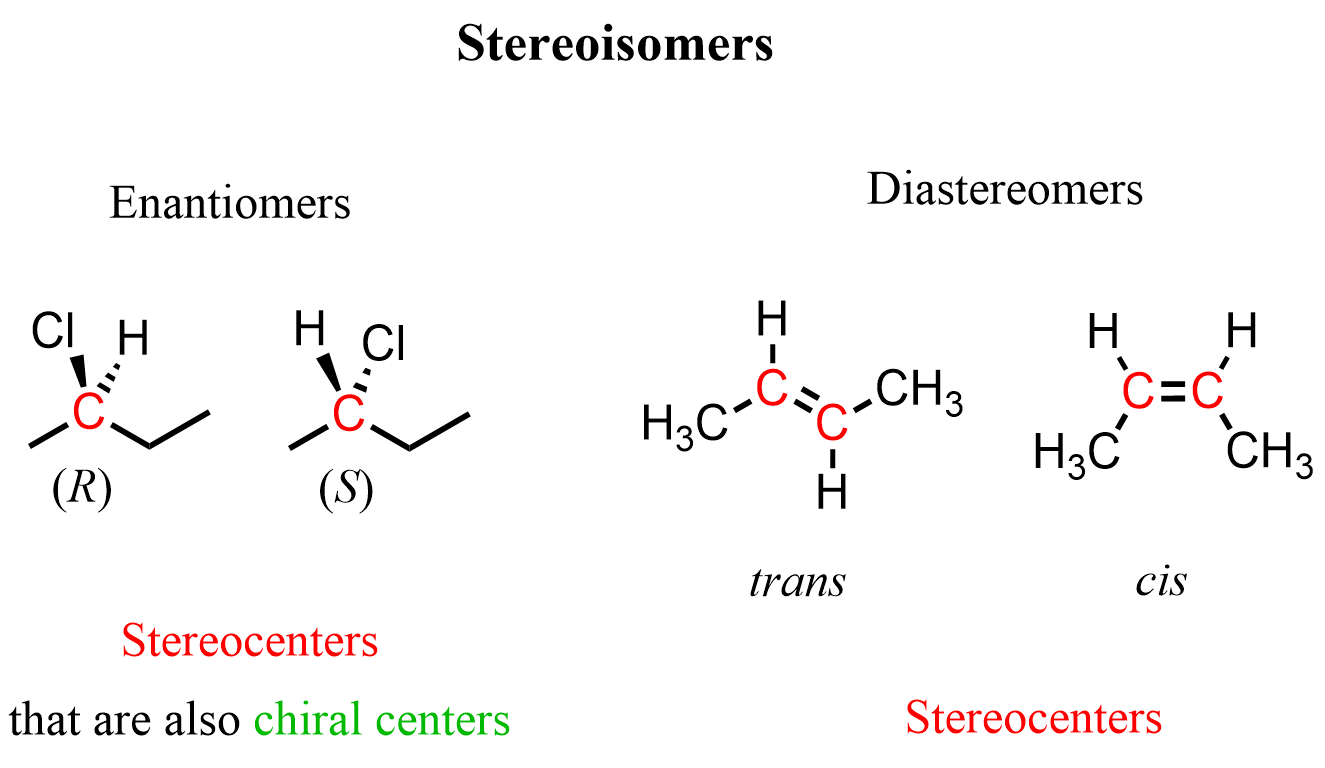
So, to summarize: all chiral centers are stereogenic, but not all stereogenic centers are chiral; stereogenic centers include any feature that produces non-superimposable stereoisomers, including double bonds.
We have a separate post on comparing stereocenters and chiral centers here, so feel free to check that for more details and examples.
Isomeric Alkenes are Diastereomers
Cis and trans isomers are also called geometric isomers, which are a type of stereoisomer. Remember from the chapter on stereochemistry that stereoisomers are compounds with the same molecular formula and the same connectivity of atoms but differ in the spatial arrangement of their atoms. This means cis and trans (geometric) isomers fall under the category of stereoisomers, and, if we want to specify further, they are diastereomers because they are not nonsuperimposable mirror images of one another.

Just like R and S configurations, the cis and trans configurations cannot be changed without breaking bonds. Therefore, both of these are configurational isomers within the category of stereoisomers. The terminology can feel overwhelming at times, and you can refer to this post for an overview and a comprehensive chart of isomerism, but for your organic chemistry class, remember: cis and trans are geometric isomers, and they are a type of diastereomer.
This distinction becomes especially important when the alkene is part of a larger molecule that may contain additional stereocenters, because then you can have multiple stereochemical relationships occurring in the same compound.
Alkenes Connected to Chiral Centers
Things become even more interesting when the alkene is attached to one or more chiral centers.
In such molecules, stereoisomerism around the double bond combines with chirality at tetrahedral centers, giving rise to diastereomers or enantiomers, depending on the arrangement.
For example, R and S 2-chlorobutanes are enantiomers because the molecules have one chiral center, and it is inverted. However, if we add a double bond to them while altering the E and Z configuration, they are no longer enantiomers – they become diastereomers.
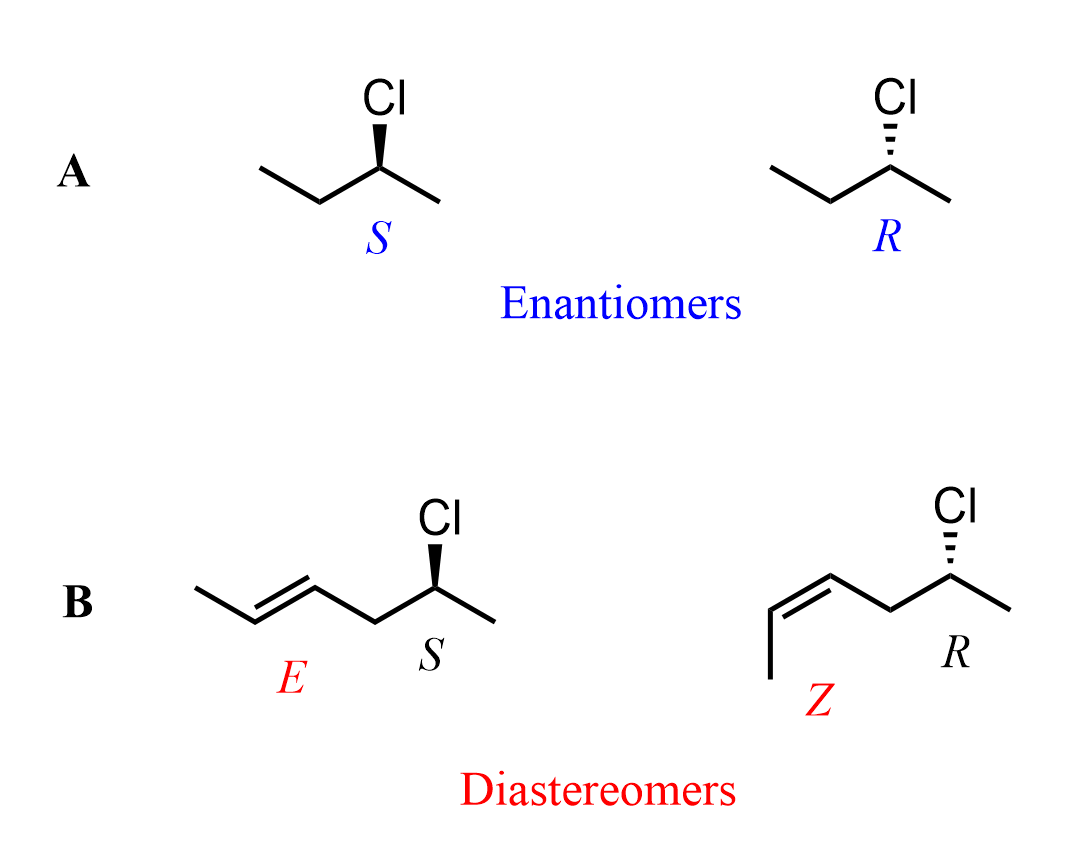
Notice that it does not matter if the chiral centers are inverted because the E and Z configurations of the double bonds make them stereoisomers that are not mirror images, i.e., diastereomers.
This does not always have to be the case, though. If the configurations of the double bonds are identical, then inverted chiral centers make the molecules enantiomers. Consider the combination of A and B in the next image:
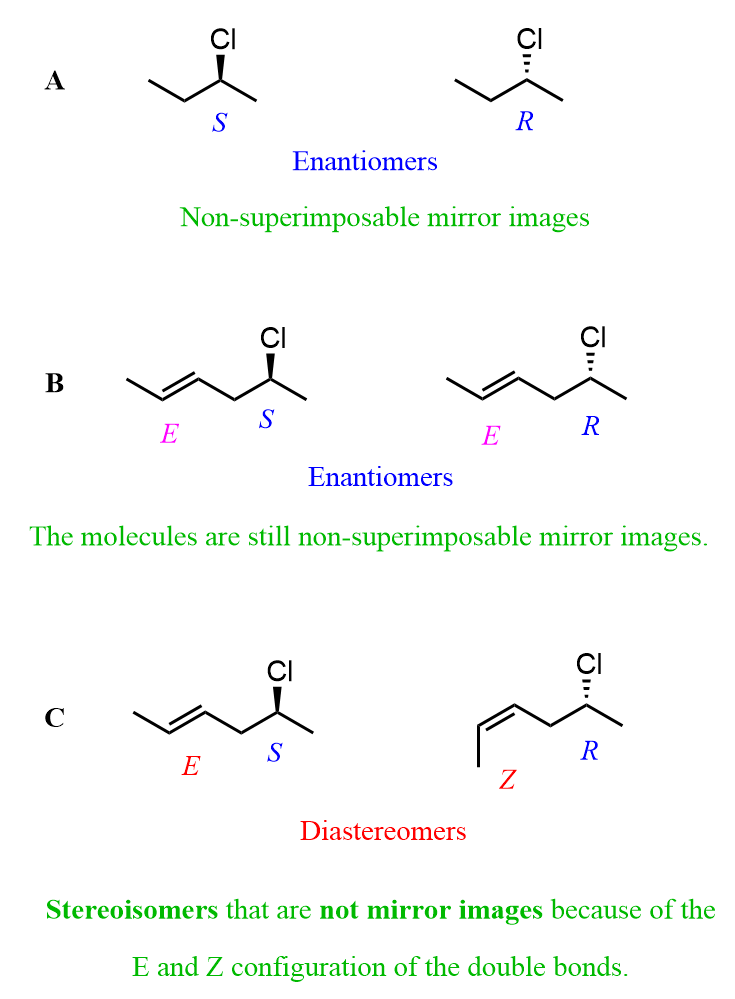
The pattern we see here is that if only the chiral center changes, the two molecules are enantiomers. On the other hand, any other combination of changing or retaining the R/S and E/Z configurations gives a pair of diastereomers. For example, in option C, both the chiral center and the configuration of the double bond are changed, which leads to a pair of diastereomers.
To generalize this pattern, let’s add another chiral center and consider the stereoisomers of 6-bromo-5-chlorohept-2-ene.
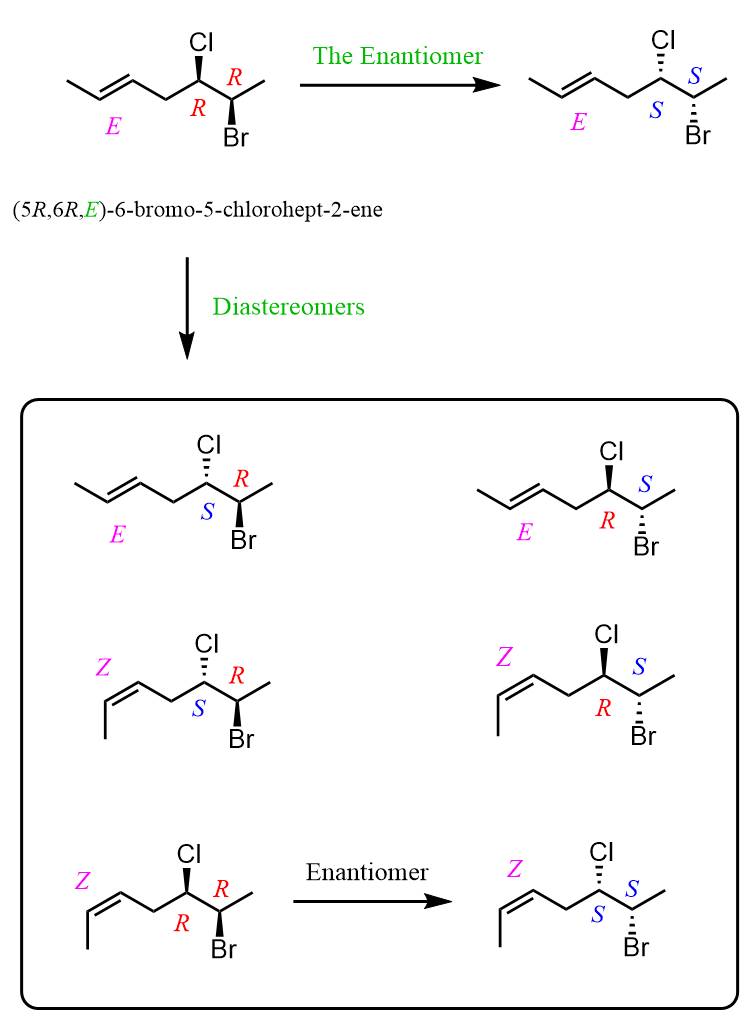
We can see that, like any other chiral molecule, it can have only one enantiomer and several diastereomers corresponding to the other possible configurational combinations of the stereogenic centers.
Summarizing the Stereochemistry of Alkenes
The stereochemistry of alkenes arises as a result of restricted rotation about the carbon-carbon double bond. This rigidity creates the possibility of geometric isomerism, where the relative positions of substituents on each side of the double bond determine whether a molecule is cis (Z) or trans (E).
When both carbons of the double bond bear different substituents, the E/Z system replaces the simpler cis/trans notation to describe the configuration unambiguously using the Cahn-Ingold-Prelog priority rules.
Cis/trans or E/Z isomers are forms of stereoisomers, and more specifically, they belong to the class of diastereomers, because they are not mirror images of each other. This becomes especially interesting when alkenes are part of molecules that also contain chiral centers – the combination of double-bond geometry and chirality can give rise to multiple stereochemical relationships, including both enantiomers and diastereomers.
If one of the carbons is connected to two identical groups, geometric isomerism is not possible, and the alkene is non-stereoisomeric.
Check Also
Naming Alkenes by IUPAC Nomenclature Rules- Cis and Trans Isomers
- R and S Configuration
- Configurational Isomers
- Enantiomers
- Diastereomers
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Newman Projection of Chair Conformation
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- The reactions of Alkenes can be found on the Organic Chemistry 1 and 2 Topics page



