The SN1 mechanism is a unimolecular process, which means only the substrate participates in the rate-determining step of the reaction. The rate-determining step is the loss of the leaving group, which forms a carbocation.
There are a few main features we need to recall about carbocations to understand the stereochemistry of SN1 reactions:
1) Carbocations are sp2-hybridized, therefore, the positively charged carbon has a planar geometry:
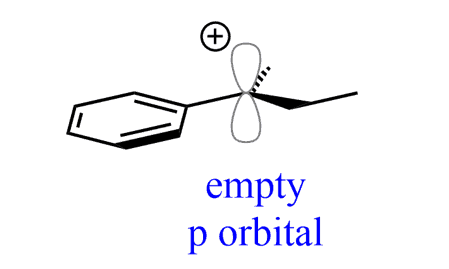
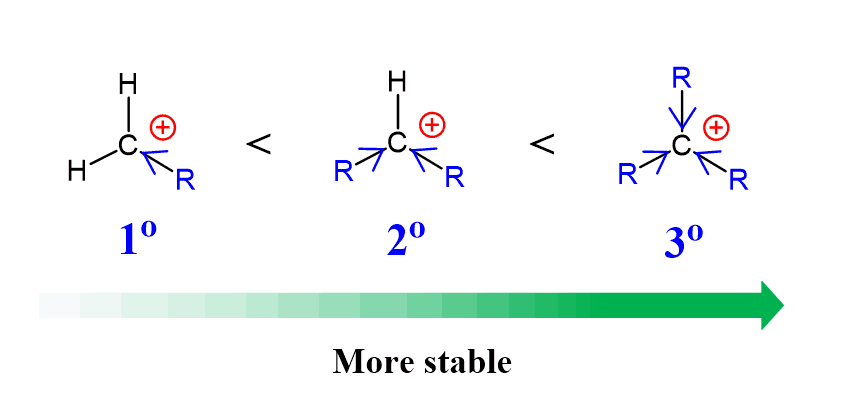
2) The stability of carbocations increases with the number of alkyl groups:
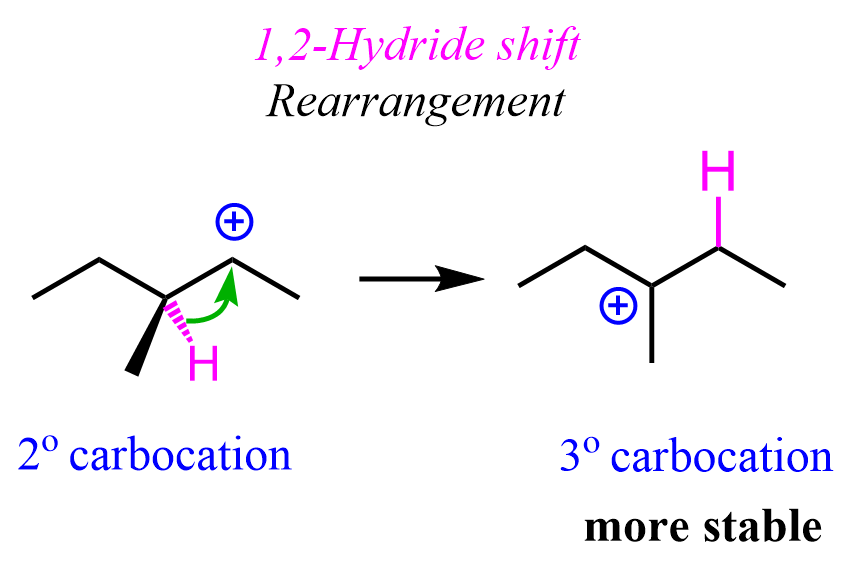
3) Whenever possible, the carbocation will undergo a rearrangement to transform into a more stable carbocation:
Here is a list of alkyl halide reactions with water with different stereochemical outcomes, and we are going to address each of these pathways.

A quick reminder of why the reaction of alkyl halides with water proceeds by the SN1 mechanism. Water is a poor nucleophile and a weak base; therefore, it only does SN1 or E1 reactions on alkyl halides. The only exception would be primary halides, which cannot undergo an SN1 reaction as the intermediate primary carbocation is way too unstable. The E1 elimination is generally favored when the reaction is heated. You can check this article for more details on deciding if the mechanism is SN1, SN2, E1, or E2.
Alkyl Halides with One Chirality Center
Let’s consider the hydrolysis of (R)-2-bromobutane, which has only one chiral center. Here, we need to remember the first property we mentioned about carbocations. That is, the positively charged carbon is sp2-hybridized and therefore it is achiral (not chiral). We also mentioned that carbocations have planar geometry (the positively charged carbon) and therefore, once formed, it is attacked from both faces of the planar carbon, giving a racemic mixture of enantiomers:
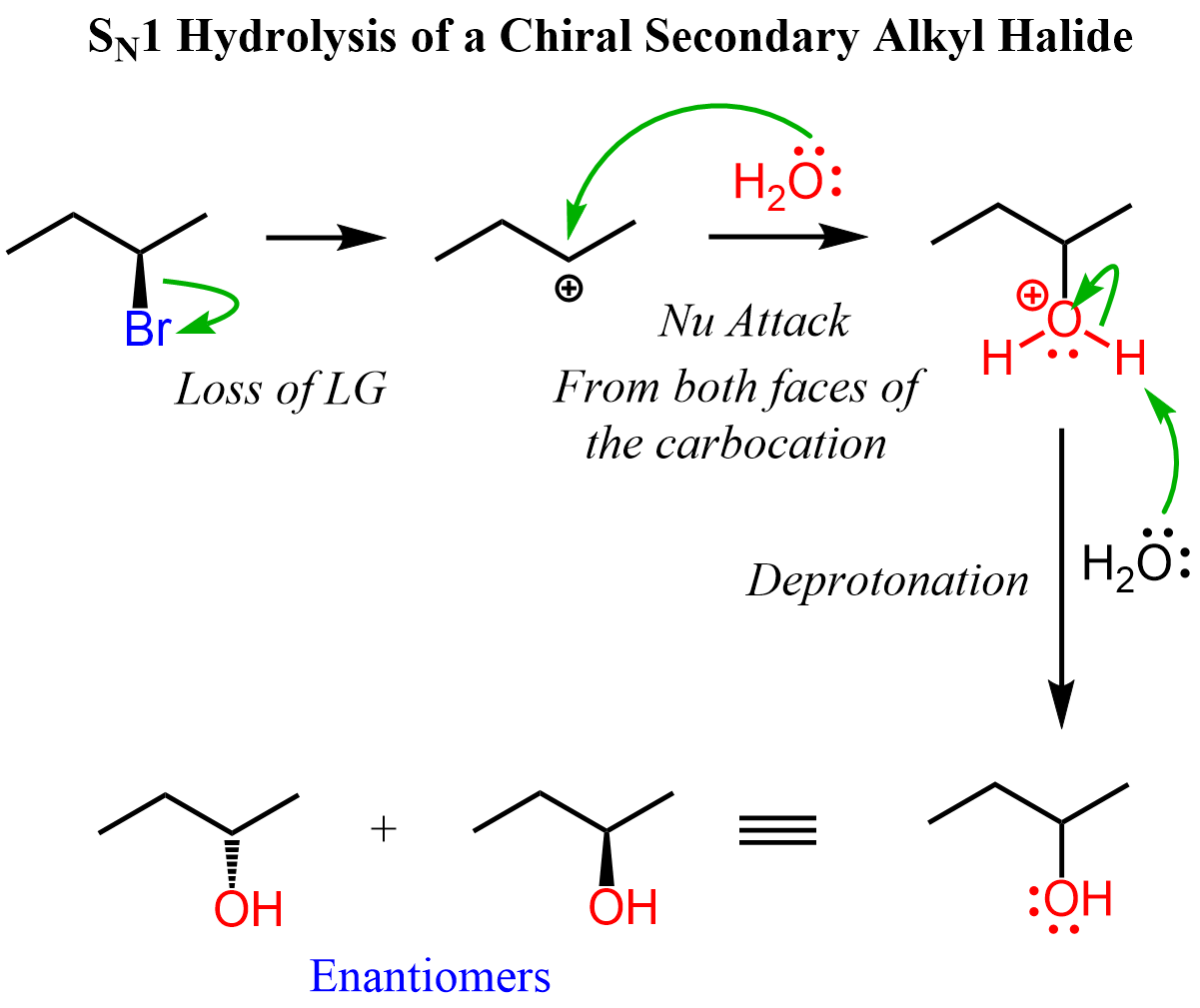
Loss of Chirality in SN1 Reactions
In the example above, we saw that from a chiral alkyl halide, a mixture of enantiomers is formed in an SN1 reaction. This is the most common stereochemical outcome you will see in SN1 reactions. One important reminder: racemic mixtures are achiral (not chiral), but they contain an equal amount of the two enantiomers.
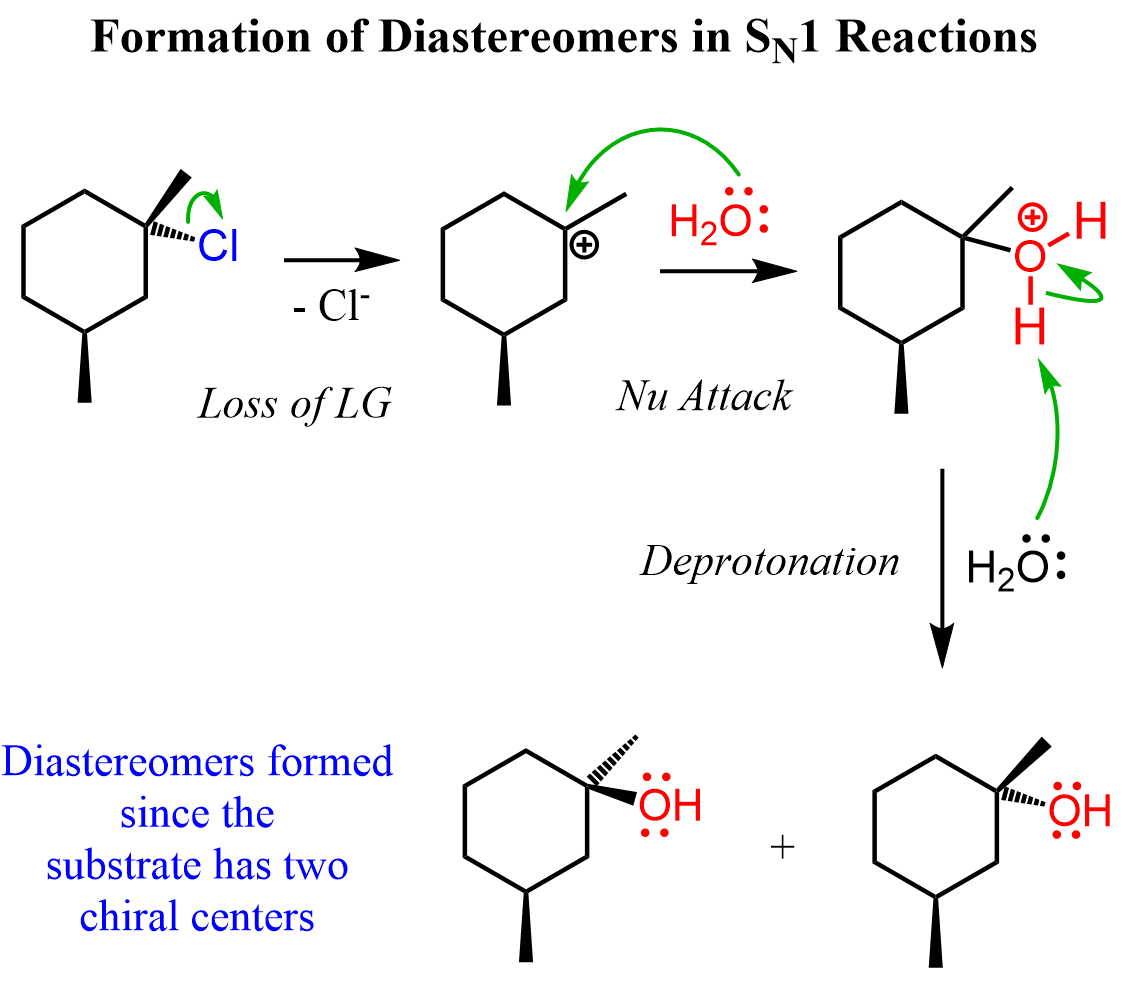
There are cases where the chiral alkyl halide is converted into a product with no chirality centers. Most often, the reason for this is a rearrangement. For example, let’s see how the substrate with two chirality centers in reaction two gives an achiral product.
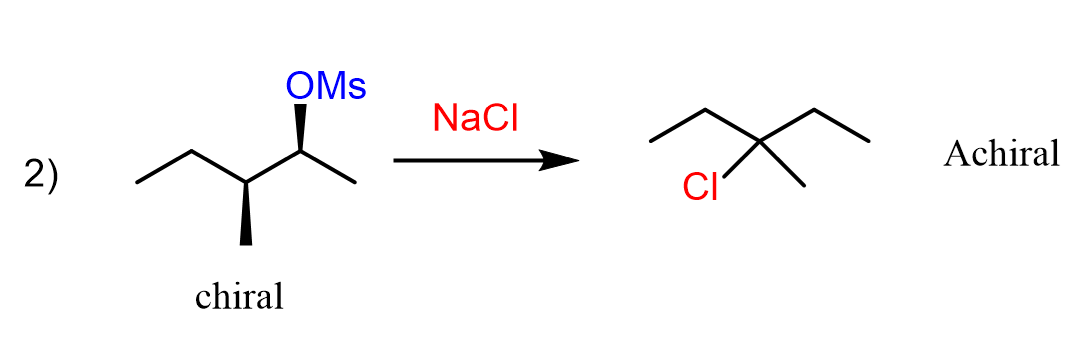
We notice from the beginning that the nucleophile in the product is not on the same carbon where the leaving group was. This should be a hint for you that there is a rearrangement happening in the reaction.
Remember, the main reason for rearrangement is the increased stability of the carbocation. Carbocations get more stable with the number of alkyl groups as they stabilize the positive charge on the carbon atom:

The mesylate substrate gives a secondary carbocation upon the loss of the leaving group. This secondary carbocation is rearranged into a more stable tertiary carbocation via a 1,2-hydride shift:
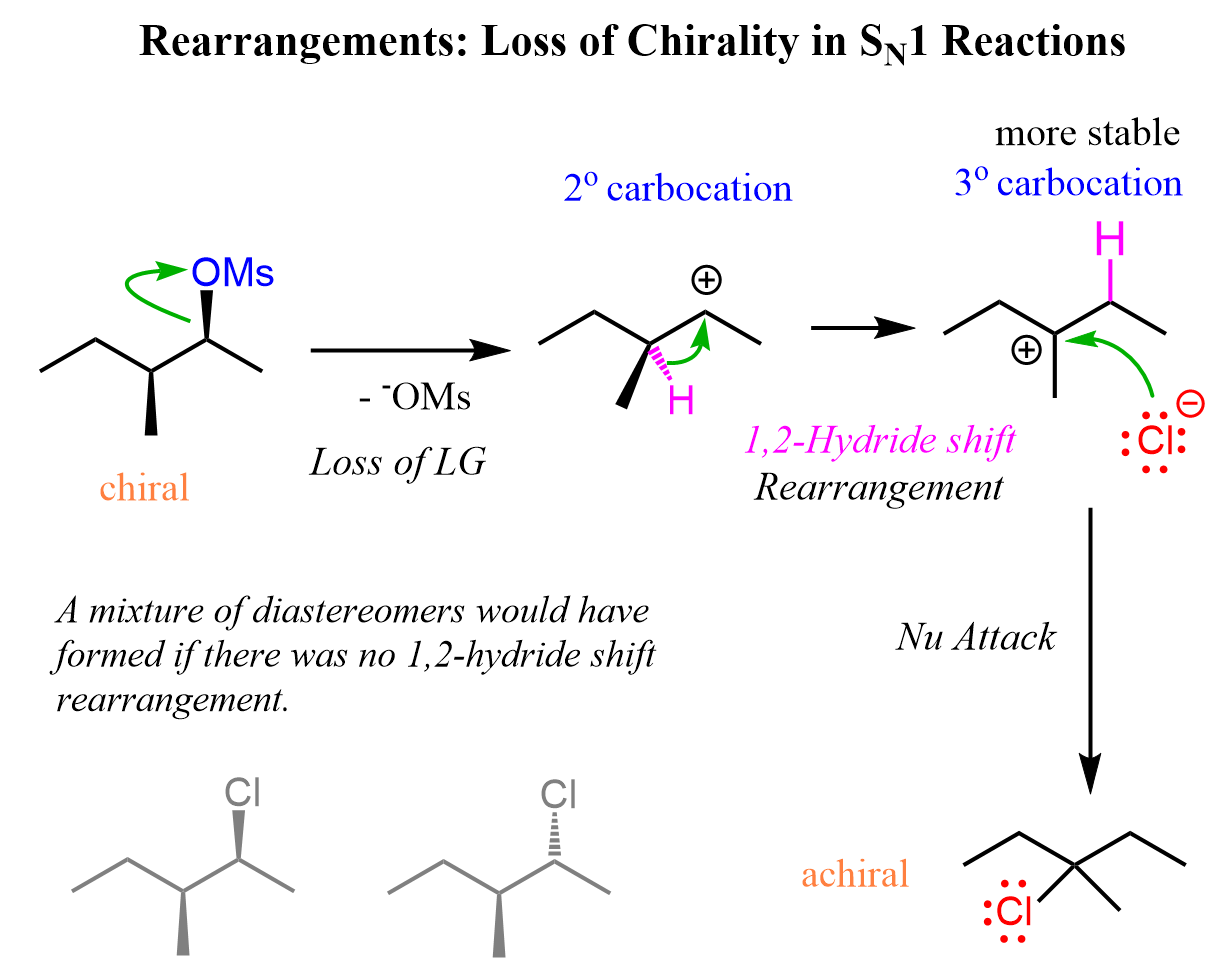
The chirality of the middle carbon is lost upon the hydride shift because carbocations are sp2-hybridized and only have three atoms connected to the positively charged carbon. Because of symmetrical structures, the nucleophilic attack of water produces an achiral alcohol.
So, keep in mind that the SN1 mechanism is a unimolecular process where a carbocation is formed in the first step. You need to always check for rearrangement possibilities when dealing with carbocations. Common mistakes when forgetting about rearrangements are wrong placement of the nucleophile in the product, or the leaving group in the substrate, picking the wrong product and etc.
Alkyl Halides with Two Chirality Centers
We saw that the nucleophilic attack on an unsymmetrical carbocation intermediate generates a mixture of both configurations, forming a mixture of enantiomers. Let’s now see what product is formed when the following tertiary alcohol is reacted with hydrochloric acid (reaction 3). Overall, the mechanism is similar to what we have been seeing so far: loss of a leaving group, followed by a nucleophilic attack. That is the pattern of SN1 reactions. However, in the case of alcohols, you need to remember that the OH group is not a leaving group (E1cB is perhaps the only notable exception, but don’t worry about it in this chapter).
In the reaction with acids, it is converted into a leaving group by protonation; therefore, the first step in the reactions of alcohols with acids is the protonation of the oxygen. After this, water is the leaving group, which is obviously very stable and therefore an excellent leaving group. The resulting tertiary carbocation is the most stable we can get from this molecule; thus, the nucleophilic attack of the chloride ion regenerates the chirality center, again with both configurations.
Notice, however, that the two products are not enantiomers – they are diastereomers, because the configuration of the wedge methyl on the bottom does not change.
It is worth mentioning that all the steps in this reaction are reversible, and the reaction is pushed forward due to the excess of the reactant. A common question from my students is how come it is the chloride and not the water attacking the carbocation. The answer is they both do; the former is simply the reverse step, generating the alcohol, which is again converted into the carbocation. It is just the excess of the halide ions that shifts the reaction forward.
Therefore, we can also convert alkyl halides to alcohols when reacted with an excess of water. Usually, it is used as a mixture with an organic solvent:
Cis and Trans Diastereomers in SN1 Reactions
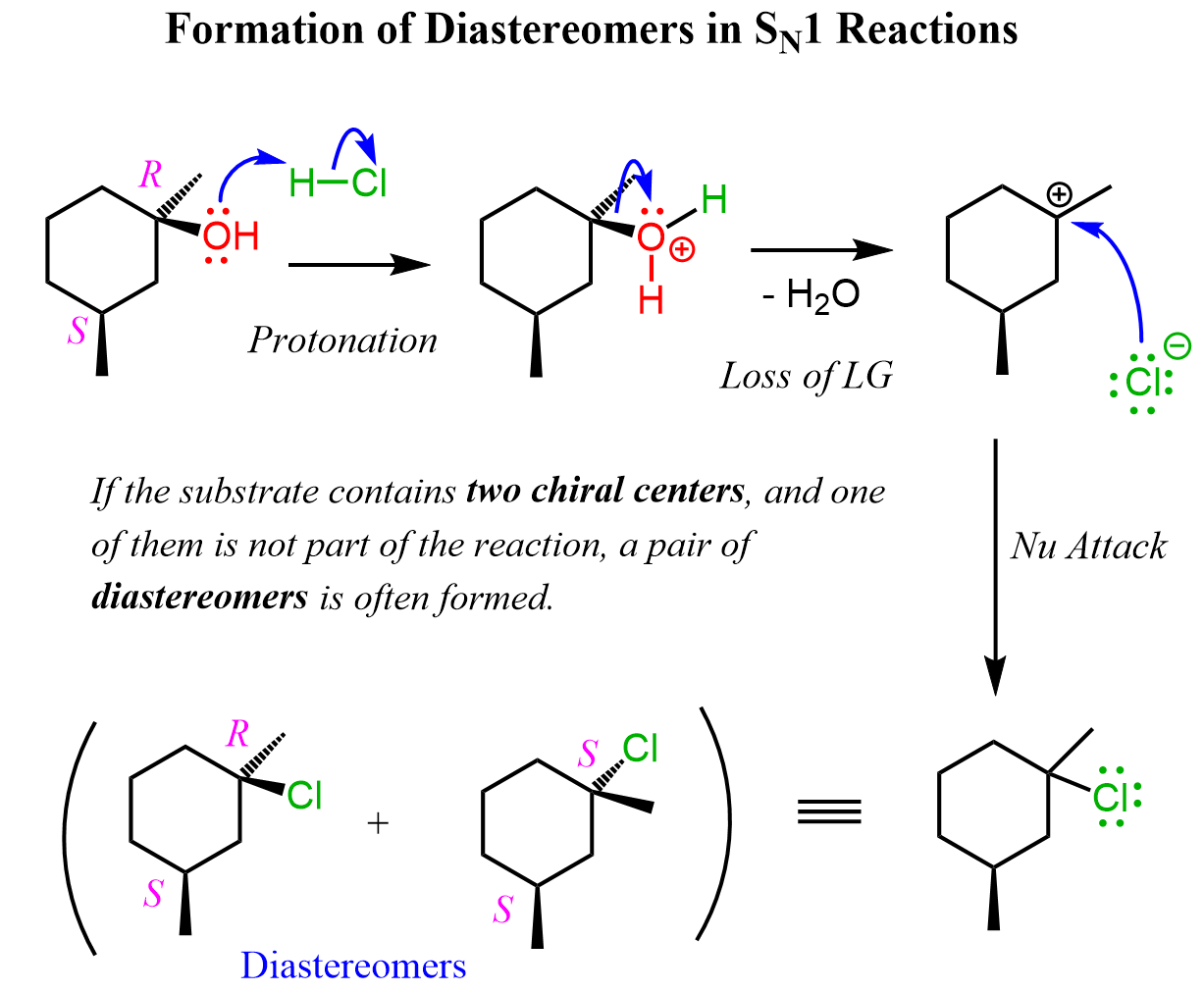
Let’s compare the products in the alkyl hydrolysis reactions 4 and 5. Like in the previous example, in reaction 4, only one of the chirality centers is part of the substitution, and therefore, a mixture of diastereomers is formed. Can you draw the mechanism of this reaction? Let’s now compare this with the alkyl halide in reaction 5.
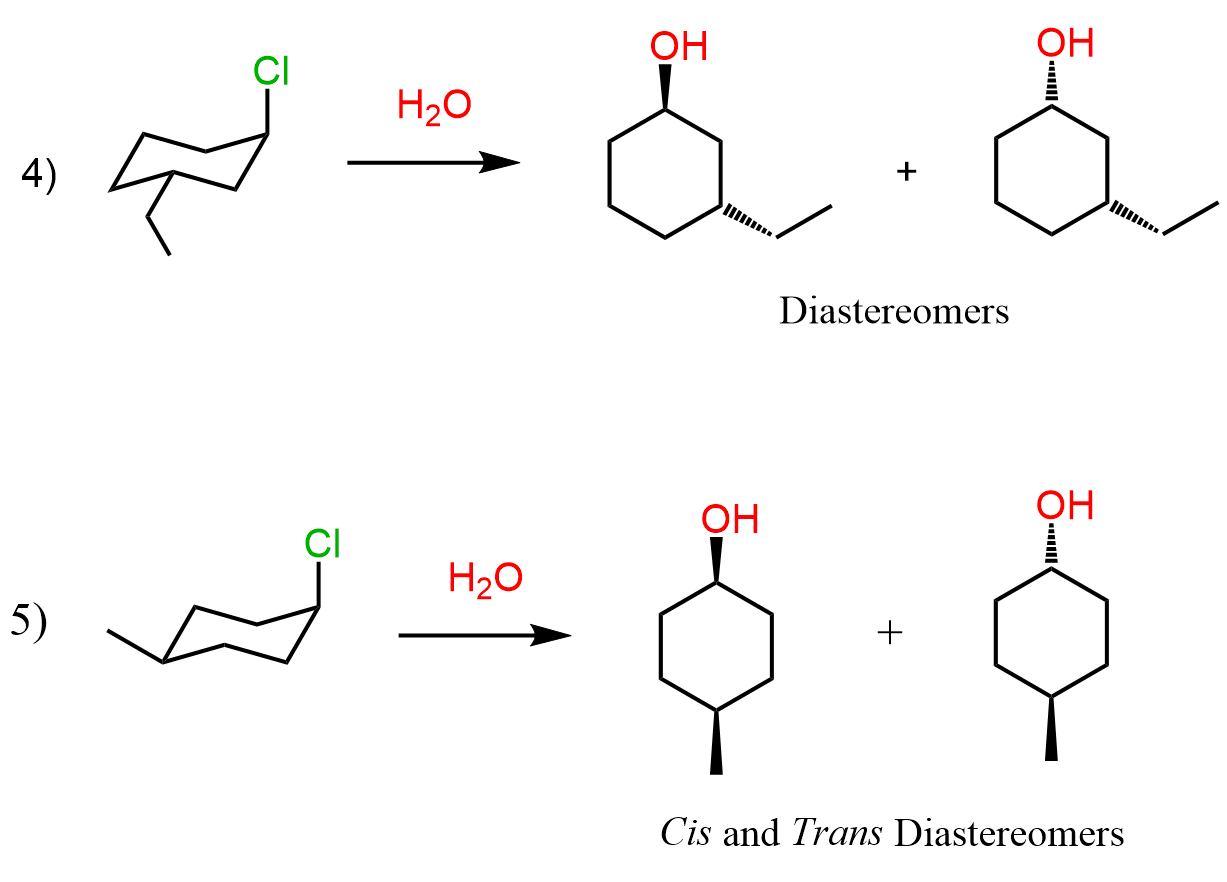
The main difference here is that the first molecule is achiral as it has a plane of symmetry. However, we cannot ignore the stereochemistry of the hydrolysis because there are two alcohols formed in that reaction. These are diastereomers because, remember, diastereomers are stereoisomers that are not mirror images. We can look at these alcohols as cis and trans isomers because of the relative orientation of the methyl and OH groups. So, keep in mind that cis and trans isomers are diastereomers even if there are no chiral centers in the given molecules.
Let’s also draw the SN1 mechanism of this hydrolysis reaction:
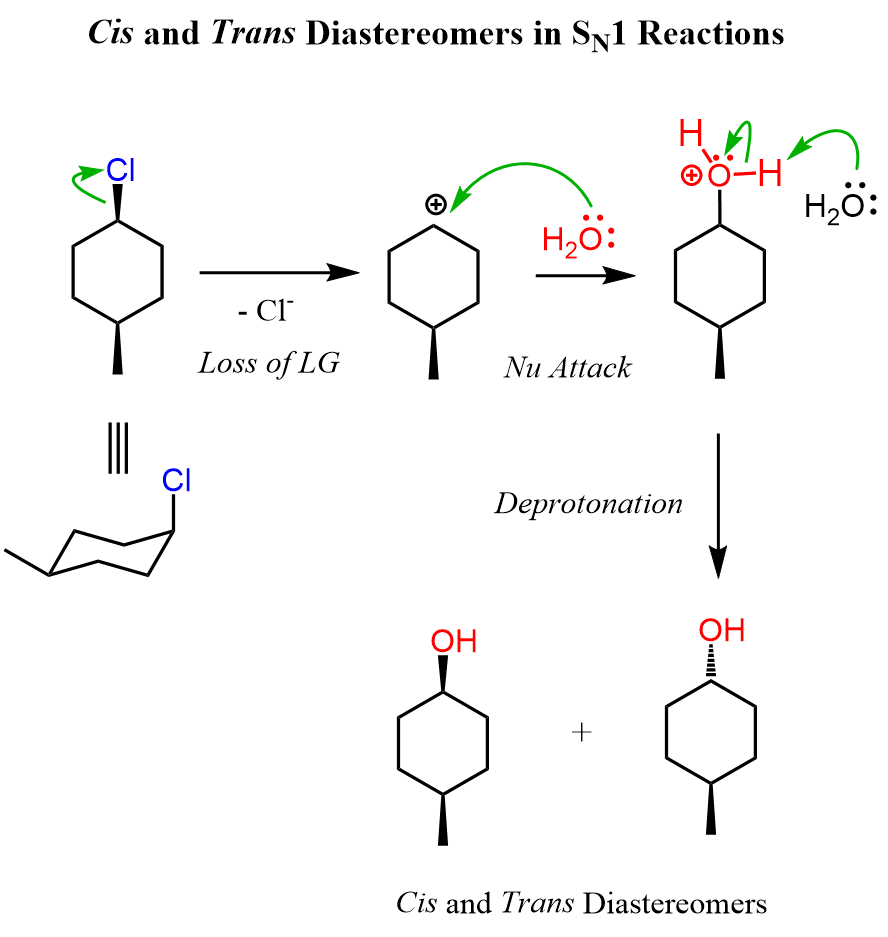
Try to draw the mechanism of reaction 4. Refer to this article for converting cyclohexane into chair conformations and vice versa.
Summarizing the Stereochemistry of SN1 Reactions
SN1 reactions proceed via racemization of the chiral center that is part of the reaction. If a new chirality center is formed during the addition of the nucleophile, a pair of enantiomers is obtained. A common exception is when the substrate already contains an additional chiral center that is not part of the reaction. In this case, a pair of diastereomers is formed.
Rearrangements will occur whenever possible, so always watch out for those when dealing with unimolecular reactions such as SN1 and E1. Check this article on the most common exceptions in SN1 and SN2 reactions.
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- The Substrate and Nucleophile in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
