Sterics or steric interactions are the obstructions caused by the size of neighboring groups and atoms. In other words, it is simply two or more atoms/molecules/groups getting in each other’s way because of the tight space.
So, how does stereics affect the SN1 and SN2 nucleophilic substitution reactions?
Let’s start with the basics: the SN1 and SN2 reactions, in fact, any reaction for that matter, require getting the reactants to come in close proximity and collide with sufficient energy. Therefore, steric hindrance must play some role in SN1 and SN2 reactions.
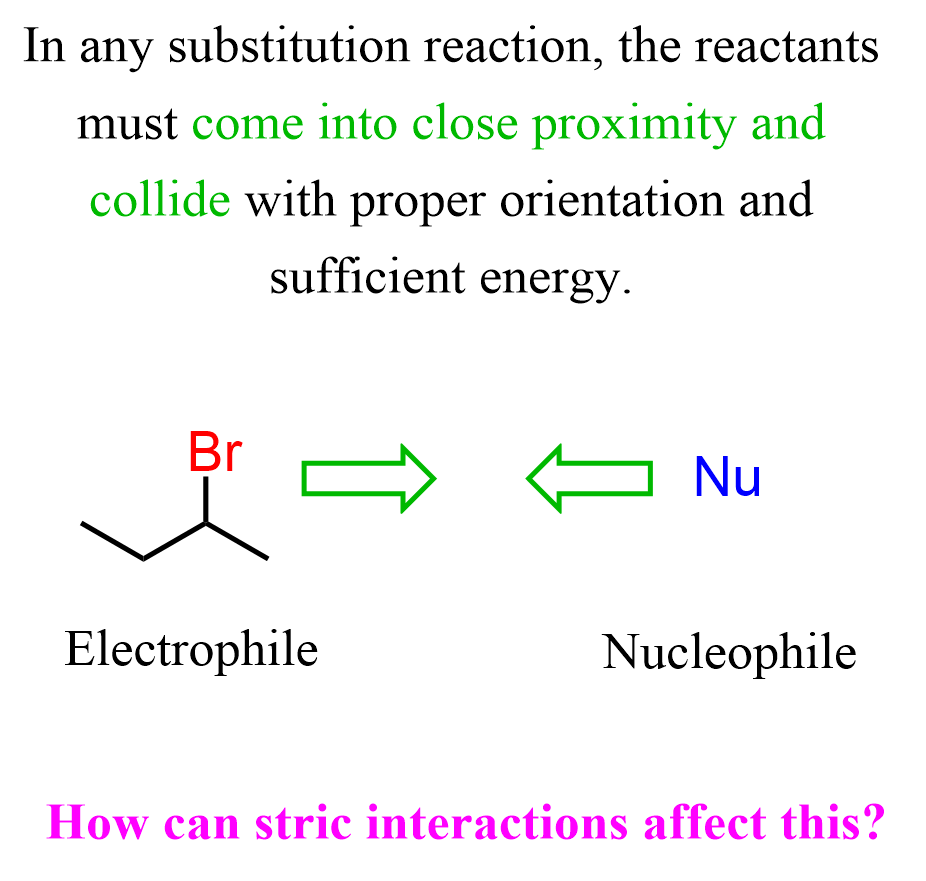
The effect is not the same on SN1 and SN2 reactions, and that is because they occur by different mechanisms.
Let’s start this comparison with the effect of steric interactions in the SN1 mechanism.
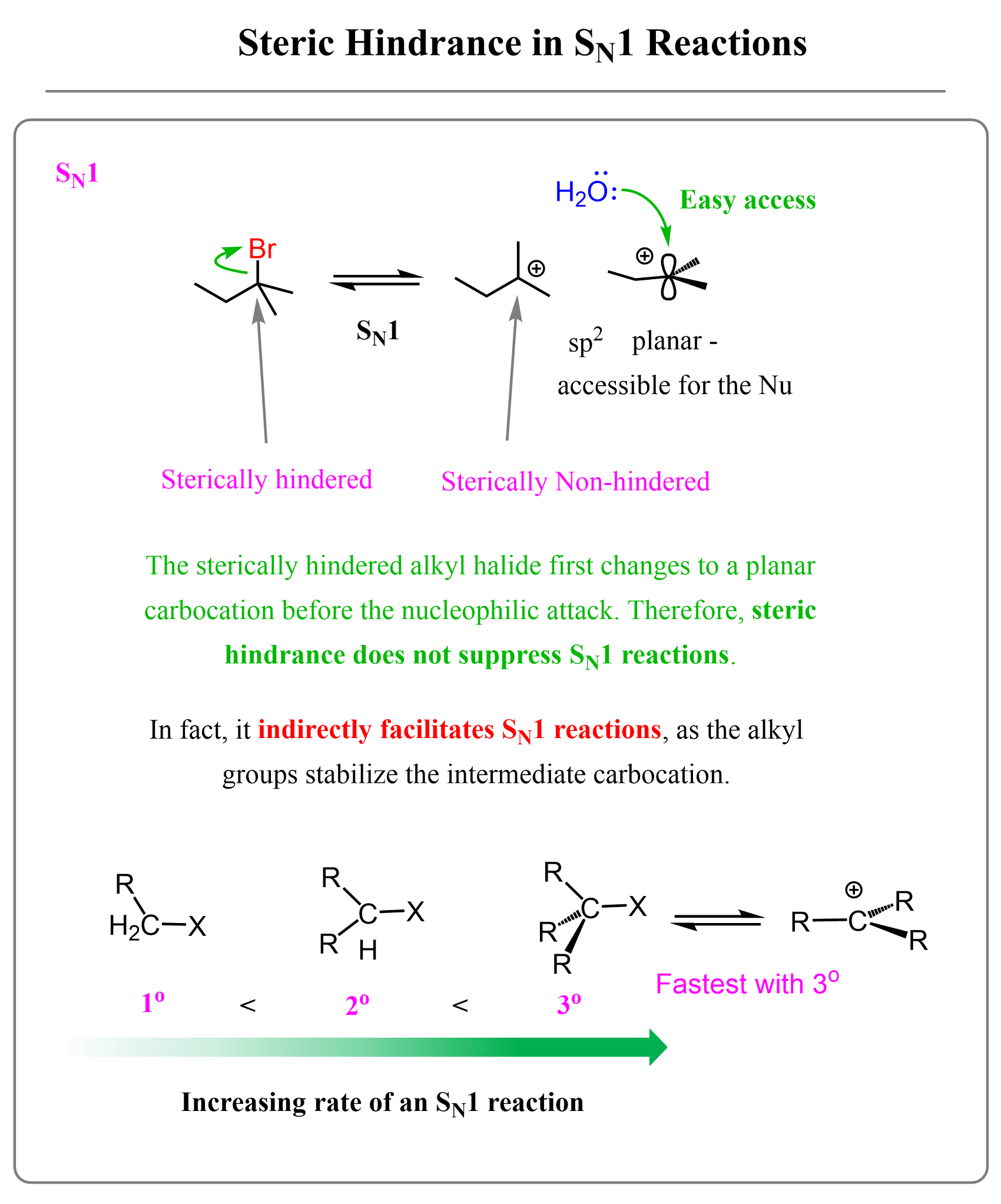
Steric Effects on SN1 Reactions
We can explain the changes in the reactivity trends of alkyl halides in SN1 and SN2 reactions by the fact that in the SN2 mechanism, the alkyl halide reacts as it is, whereas in SN1, it first converts into a planar (flat carbon) intermediate – a carbocation.
Now, what do we mean exactly by “the structure is as it is”? If we recall the SN1 mechanism, we know that the alkyl halide is first “broken down” by loss of the leaving group, forming a carbocation intermediate, and only after this, the nucleophilic attack takes place:

This is a big difference because the loss of the leaving group makes the electrophilic carbon more accessible, as it is planar and sp²-hybridized. Which means, the steric hindrance is not a detrimental factor in SN1 reactions. In fact, it indirectly helps this mechanism because having many alkyl groups increases the stability of the intermediate carbocation. Remember, carbocation stability increases with the number of alkyl groups because of their electron-donating inductive effect:
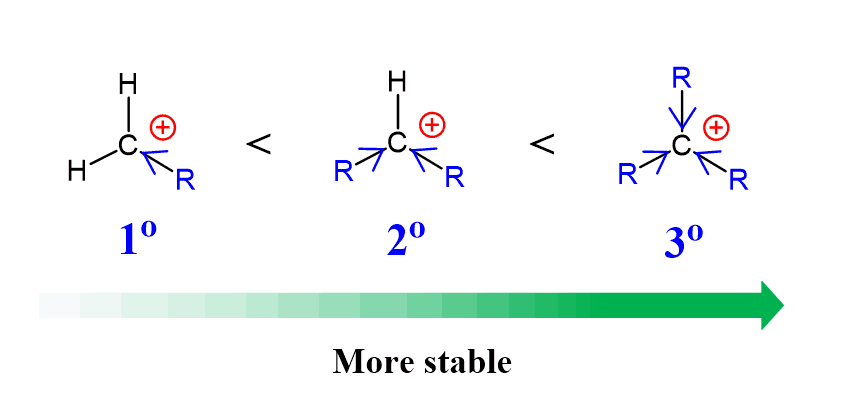
Another way alkyl groups stabilize the positively charged carbon atom is through hyperconjugation. Hyperconjugation is the charge-stabilization by pushing some electron density of the adjacent σ bond to the empty p orbital of the carbocation:

The more alkyl groups, the more C-H bonds donate electron density to the empty p orbital of the carbocation, making it more stable.
So, once again, steric hindrance by itself does not promote the SN1 mechanism. However, the bulky alkyl groups that make the substrate sterically hindered also stabilize the intermediate carbocation, which in turn accelerates the reaction.

Remember, the rate-determining step in the SN1 mechanism is the loss of the leaving group, forming the carbocation intermediate.
All of this was mentioned to remind us how the SN1 mechanism works, but the correlation with steric hindrance is that steric hindrance does not suppress the reactivity of alkyl halides in SN1 reactions because the alkyl halide is first transformed into a planar carbocation, which is very electrophilic and easily accessible by the nucleophile.
So, in a way, we can say that it is not the alkyl halide itself that reacts with the nucleophile but rather the intermediate carbocation. Therefore, it does not matter how sterically hindered the substrate is – the alkyl groups stabilize the carbocation, thus making the overall reaction faster.
Let’s summarize the effect of steric hindrance on SN1 reactions by combining the patterns in one image:
Steric Effects on SN2 Reactions
Unlike in the SN1 mechanism, the presence of alkyl groups on the electrophilic carbon makes it less accessible in SN2 reactions. This is because, in SN2, the alkyl halide reacts with the nucleophile as it is, whouth going going through structural changes caused by the loss of the leaving group, like we saw in the SN1 mechanism.
Therefore, the more substituted alkyl halides tend to react more slowly or not at all by the SN2 mechanism.
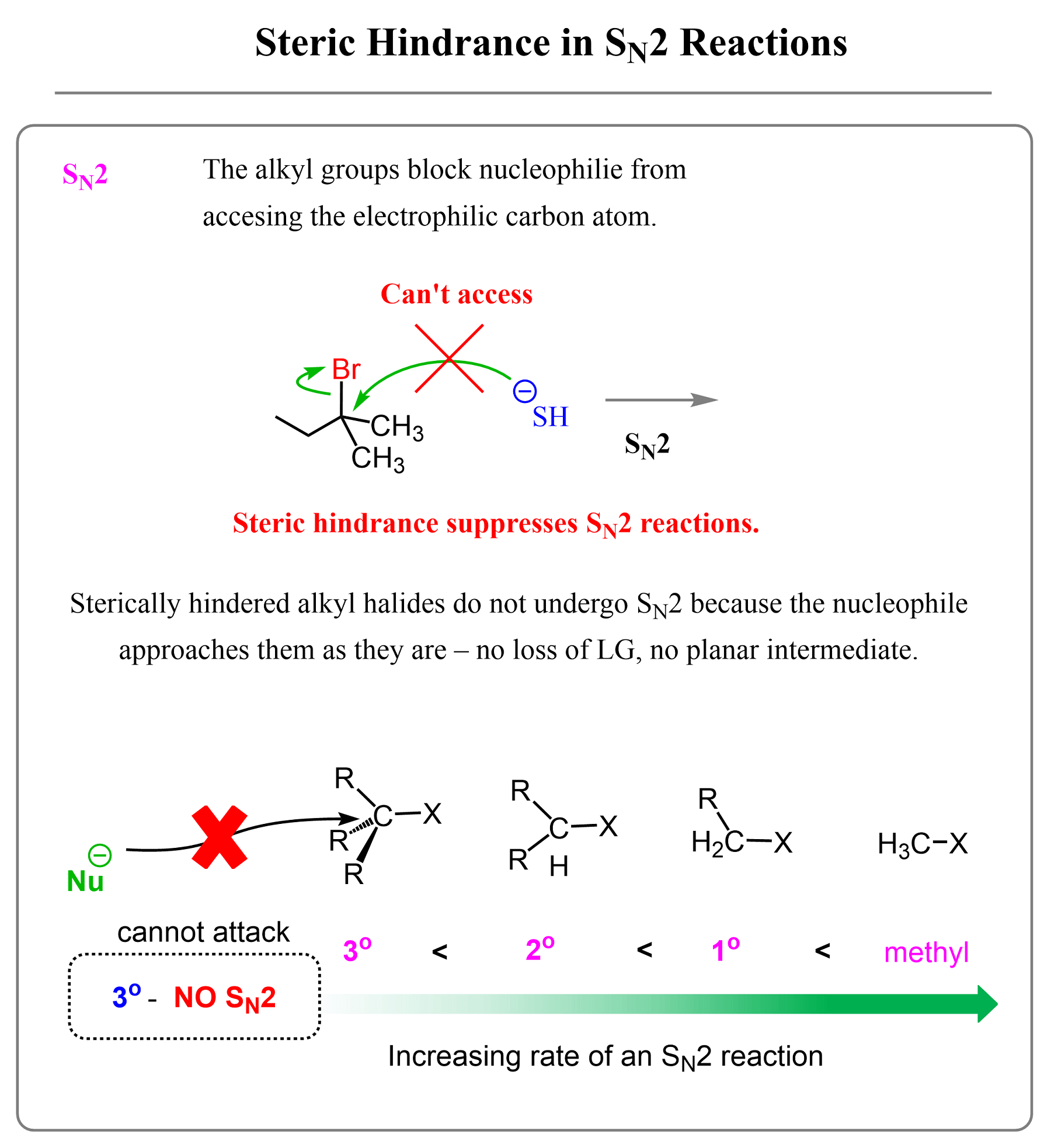
Combining these trends, you need to remember the extreme cases of alkyl halides in SN1 and SN2 reactions:
- Tertiary alkyl halides never react by the SN2 mechanism.
- Primary alkyl halides never react by the SN1 mechanism.

Other Factors Favoring SN1 or SN2 Mechanisms
Aside from the structure of the alkyl halide, we also need to evaluate the nucleophile. This is a topic that deserves a separate discussion; however, for quick reference, remember the following:
- Good/strong nucleophiles perform SN2 reactions.
- Weak nucleophiles perform SN1 reactions.
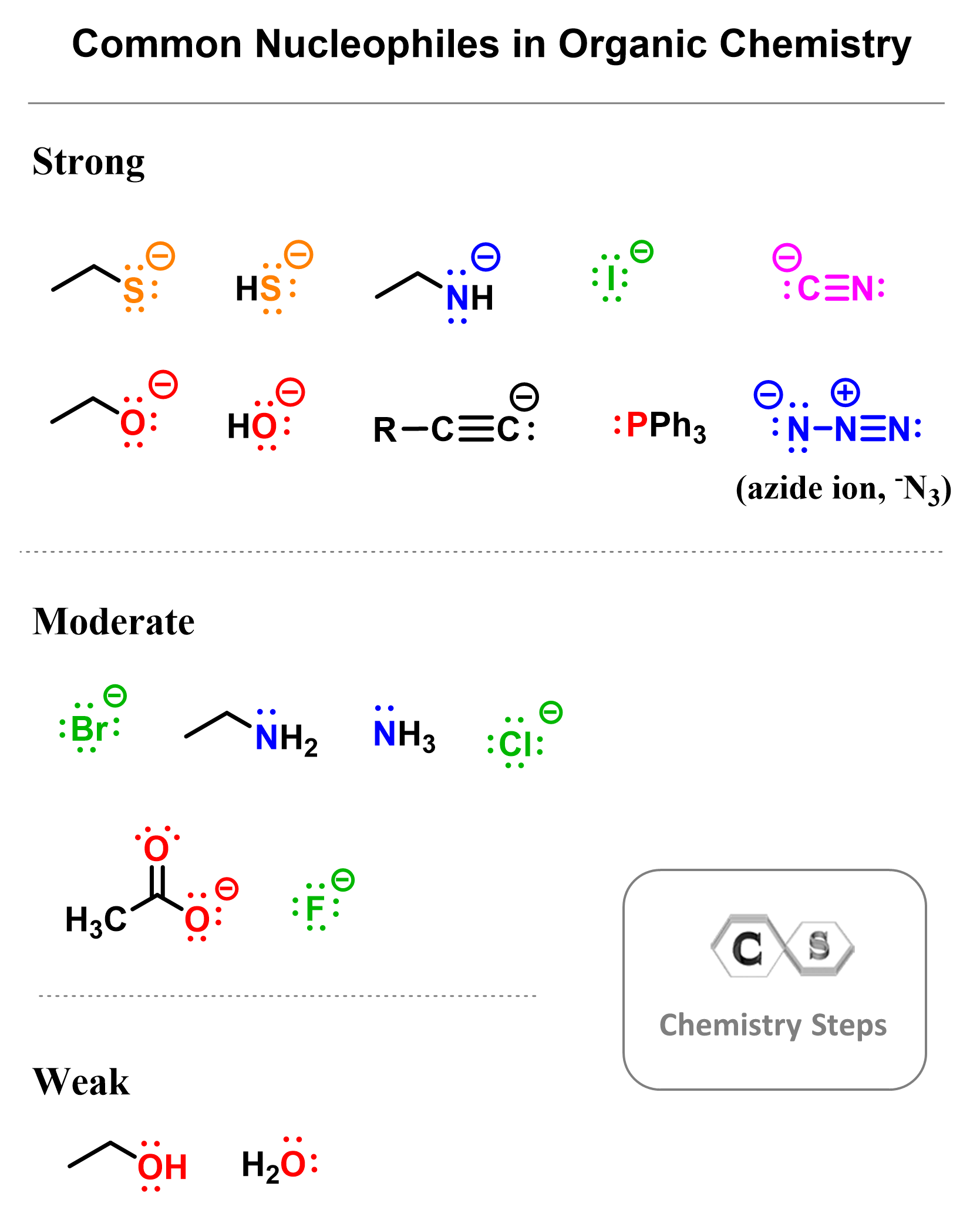
Here are the most common good nucleophiles, and for the weak ones, remember that these are mostly water and alcohols:
The use of polar protic and aprotic solvents is an additional factor that can be altered to favor the SN1 or SN2 mechanism. In short, you need to know that:
Polar aprotic solvents favor SN2 because they do not hydrogen bond with the nucleophile, allowing it to remain reactive.
Polar protic solvents favor SN1 because they stabilize both the carbocation and the leaving group via hydrogen bonding and ion-dipole interactions.
Check the linked article for a more detailed explanation of how polar protic and aprotic solvents affect the SN1 and SN2 reactions.
Below are some practice problems on the mechanism and deciding between SN1 and SN2 reactions. Feel free to check the articles mentioned after these problems if you are not familiar with any of the concepts.
Keep in mind that there is also a competition between substitution and elimination reactions, which again is decided based on the nature of the nucleophile or base and the alkyl halide.
Check these articles for a more comprehensive coverage of the competition between SN1, SN2, E1, and E2 reactions.
Deciding between SN1, SN2, E1 and E2 Reactions


