Gauche conformation is a staggered conformation, and the name is associated with gauche interactions between methyl or other alkyl groups at a 60-dihedral angle. As a general representation, compare it with the anti conformation where the large groups are at 180o:

Gauche conformations are best represented with Newman projections of butane, as there are two methyl groups on neighboring carbons:
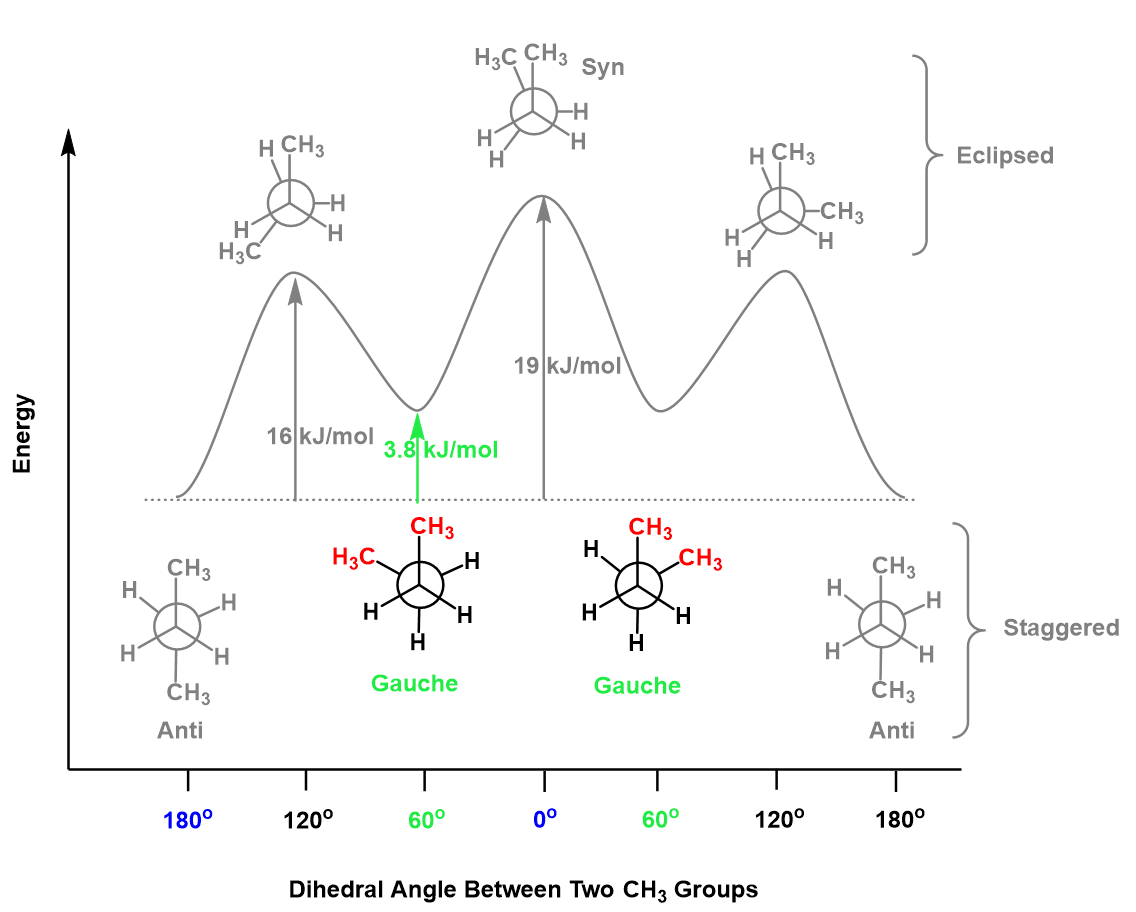
Notice that the two gauche conformations are higher in energy (less stable) than the anti conformation, and this is due to the gauche interaction between the methyl groups. Gauche interaction is a type of steric interaction, which is the obstruction caused by the size of neighboring groups and atoms. The energy associated with the gauche interaction of two methyl groups is 3.8 kJ/mol:

Notice that there are also torsional destabilizations, and these are a result of repulsive forces between the bonding electrons of neighboring groups when they are aligned (0o dihedral angle).
You can see the gauche conformations and the energy associated with them, together with other conformations, on an energy diagram represented in the following short video clip:
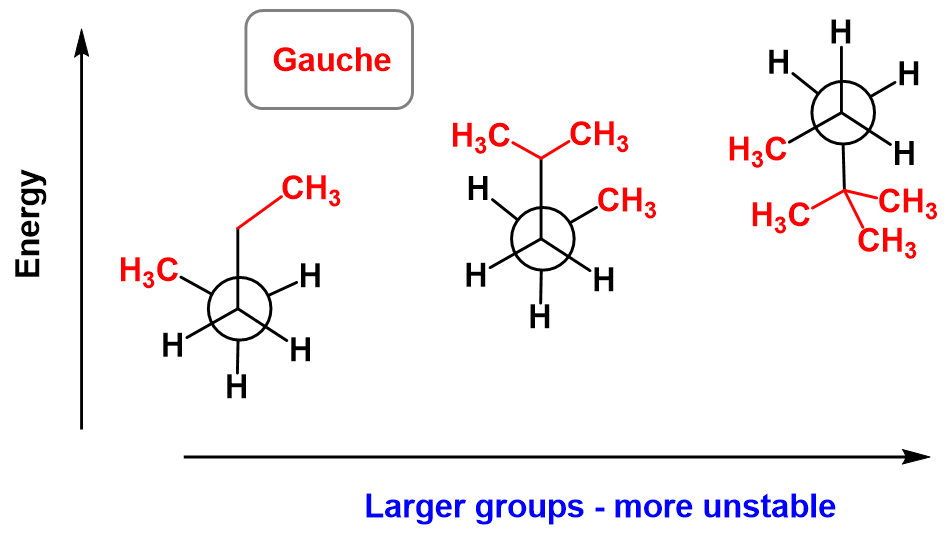
Gauche interactions are possible for any other alkyl groups, and they increase (more unstable conformation) with the size of these groups.
The gauche interaction is also responsible for the instability of axial substituents in the chair conformation of cyclohexanes. For example, notice how the axial CH3 group is in a gauche orientation to the CH2 carbon of the ring. As a result, the hydrogen atoms on those carbons experience steric strain, thus decreasing the stability of the given chair conformation. These are known as 1,3-diaxial interactions:
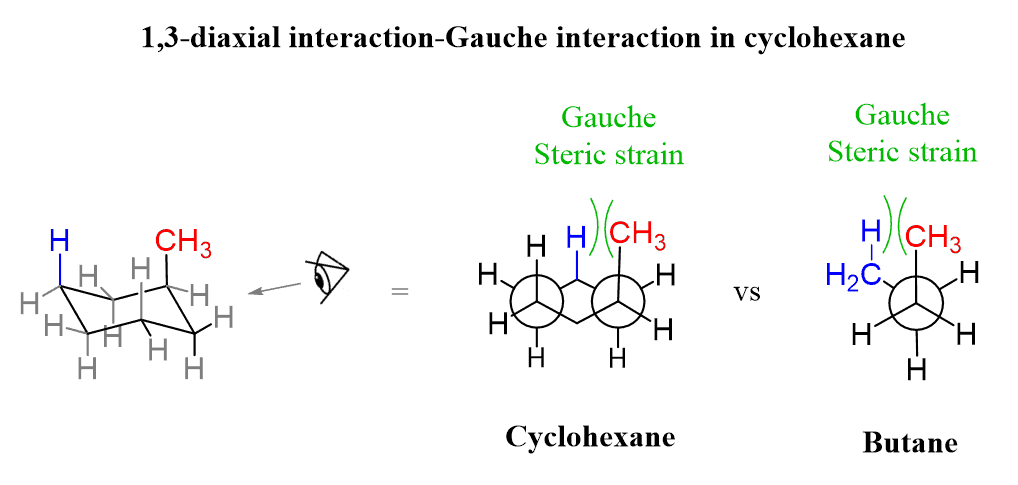
The second gauche interaction can be seen by looking from the bottom left corner:

So, the 1,3-diaxial notation is the most common way we refer to the gauche interactions of axial groups in the chair conformations. Generally, the axial conformation of a given cyclohexane is less stable than the corresponding equatorial conformation.
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
