Although quite similar and often used interchangeably, steric and torsional interactions have different definitions. Sterics or steric interactions are the obstructions caused by the size of neighboring groups and atoms. In other words, it is simply two or more atoms/groups getting in each other’s way because of the tight space.
Torsional interactions result from repulsive forces between the bonding electrons of neighboring atoms.
For example, when two hydrogen atoms on neighboring carbons are aligned, there is no steric interaction because the atoms are too small. There is, however, some unfavorable energy that is due to the torsional strain. Two methyl groups, on the other hand, are large enough to exhibit steric interactions. We can see this by comparing the eclipsed conformations of ethane and butane:
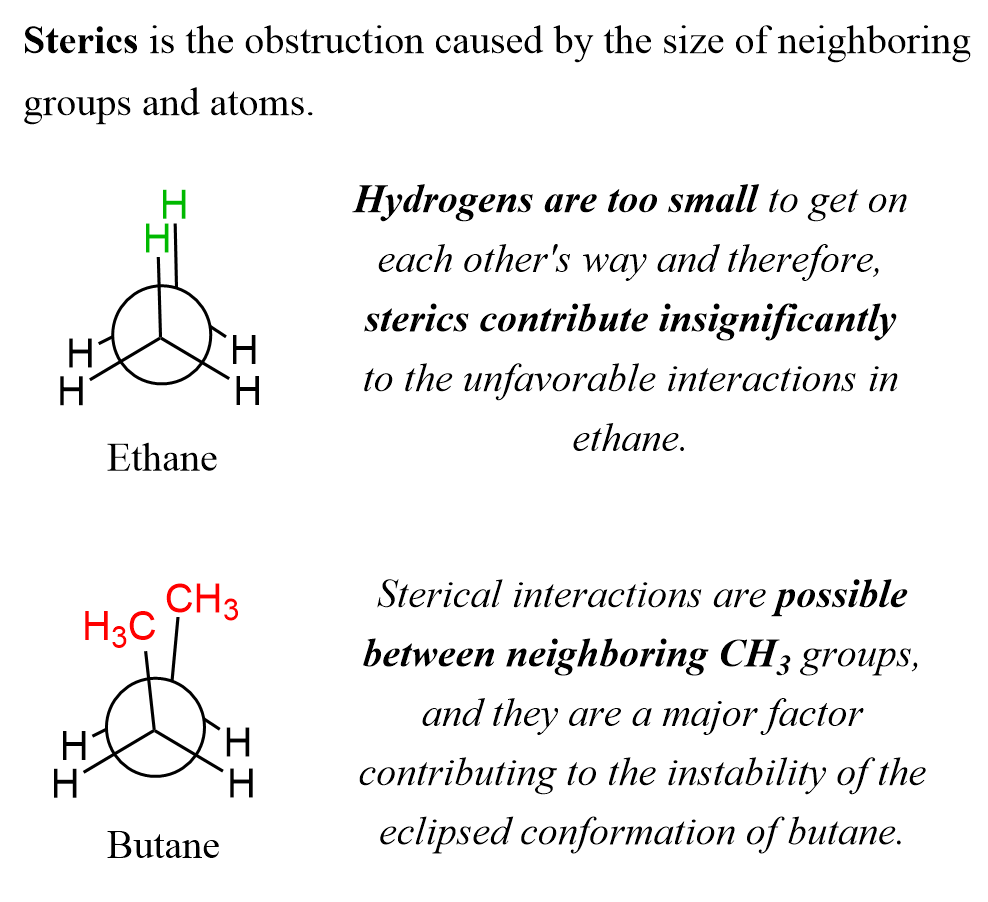
So, in ethane, when the hydrogens are eclipsed (facing each other), it is the bonding electrons that repel each other, whereas in butane, in addition to this torsional strain between each eclipsing bond, there is also a steric interaction between the methyl groups:

Here is also a short video clip to visualize better the steric and torsional interactions in the eclipsed conformations of butane:
The energy values of the steric and torsional interactions between eclipsing H and CH3 groups are given in the table below:

Torsional strain is also important in cycloalkanes because different conformations place hydrogens in an eclipsed conformation, which affects the stability of the molecules. For example, cyclobutene adopts a slightly bent geometry, also known as puckered conformation, to reduce the torsional strain caused by eclipsing hydrogens in the planar conformation:
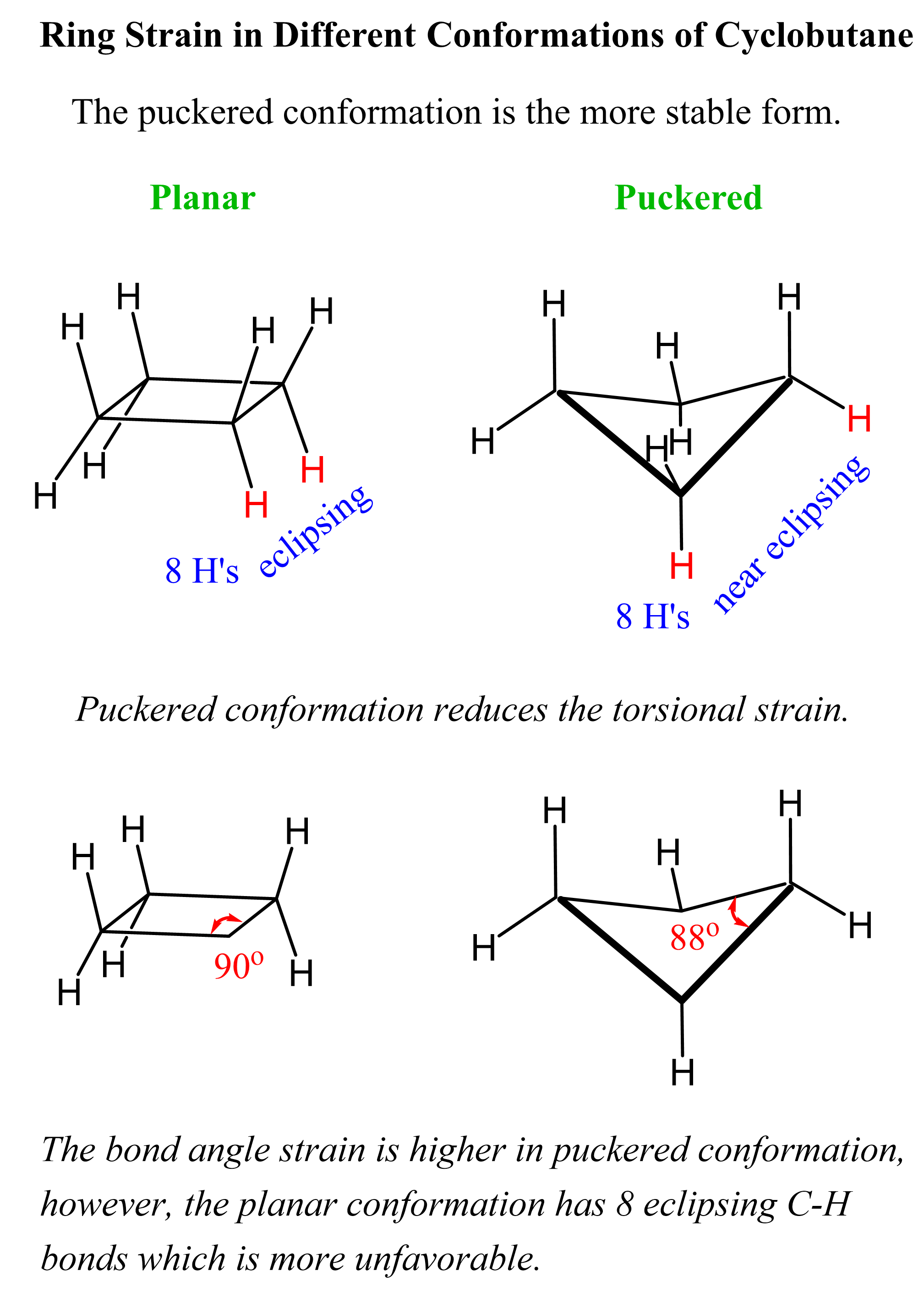
The same effect can be seen in cyclopentane, which adopts an envelope conformation to avoid some of the eclipsing hydrogens responsible for the torsional strain:
For a more detailed discussion about the conformation and stability of cycloalkanes, check the article “Ring Strain”.
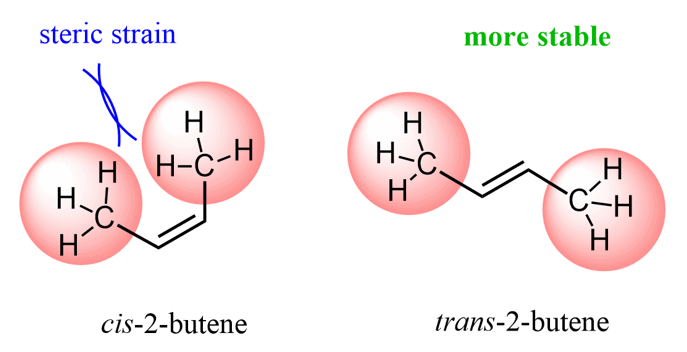
The terms sterics, steric hindrance, or steric interactions are far more common in organic chemistry because organic molecules are generally larger. For example, alkenes can often be categorized as cis and trans, and trans isomers are generally more stable because of the steric interactions in the cis isomers:
Besides intramolecular interactions, steric effects are also crucial in organic reactions. How easily molecules can approach each other often determines the reaction pathway
The classic example you will be seeing in your organic chemistry class is the factor of sterics in substitution and elimination reactions. When there are many alkyl groups on a carbon atom, it is said to be sterically hindered, and this has an effect on the course of reactions the molecule can undergo.

Don’t worry if some of these terms, like steric hindrance, nucleophilic substitution, or elimination, feel unfamiliar right now. These reactions are covered in a later chapter, and we have dedicated articles to address them in detail when the time comes.
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Stability of Cycloalkanes
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
