In this article, we will discuss the main limitations and undesired pathways in electrophilic aromatic substitution reactions and the strategies to overcome them.
Friedel-Crafts Reactions
The Friedel-Crafts reactions are used for alkylation and acylation of aromatic compounds.
Rearrangement in Friedel-Crafts Reactions
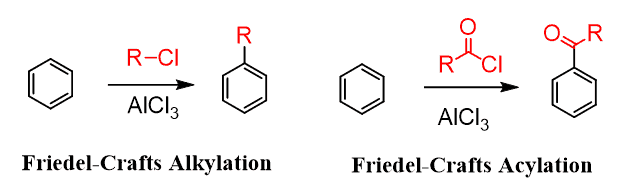
The first limitation you need to keep in mind for the alkylation reactions is that Friedel–Crafts alkylation involves a carbocation which, remember, can undergo rearrangement reactions by hydride or methyl shift. For example, in the following reaction, a secondary alkyl halide connects to the aromatic ring through a tertiary carbon because of a hydride shift:
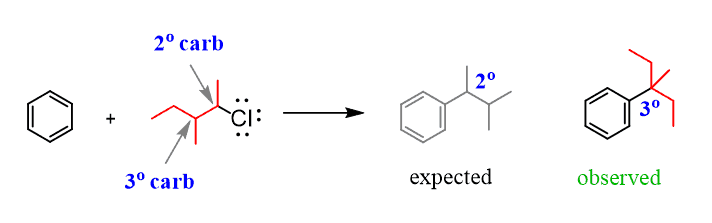
Rearrangements affect the Friedel–Crafts alkylation even when primary alkyl halides are used and no free carbocations are initially formed:

Friedel-Crafts for Aryl and Vinyl Halides
Another thing you need to keep in mind for Friedel-Crafts alkylations is that they do not work for aryl and vinyl halides because of the instability of the corresponding carbocations.

Friedel-Crafts alkylations and acylations are the slowest in electrophilic aromatic substitution reactions, so they do not work when a strongly deactivating group is present:
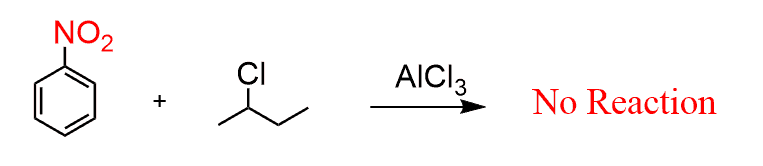
Friedel Crafts Reactions of Aniline and Phenol
Even though the amino group activates the benzene ring, and many electrophilic aromatic substitution reactions are faster compared to those with benzene, there are also quite a few that do not work as expected. The reason for this is that aniline is a Lewis base because of the lone pairs on the nitrogen. So, how does the lone pair become an obstacle in the EAS reactions of aniline? Let’s address this by discussing the Friedel-Crafts reactions, which are among the slowest of EAS, and require a Lewis acid catalyst such as AlCl3, FeBr3, etc.
The problem is that when the Lewis acid catalyst is added, it binds to the nitrogen, creating a species where the nitrogen is positively charged:
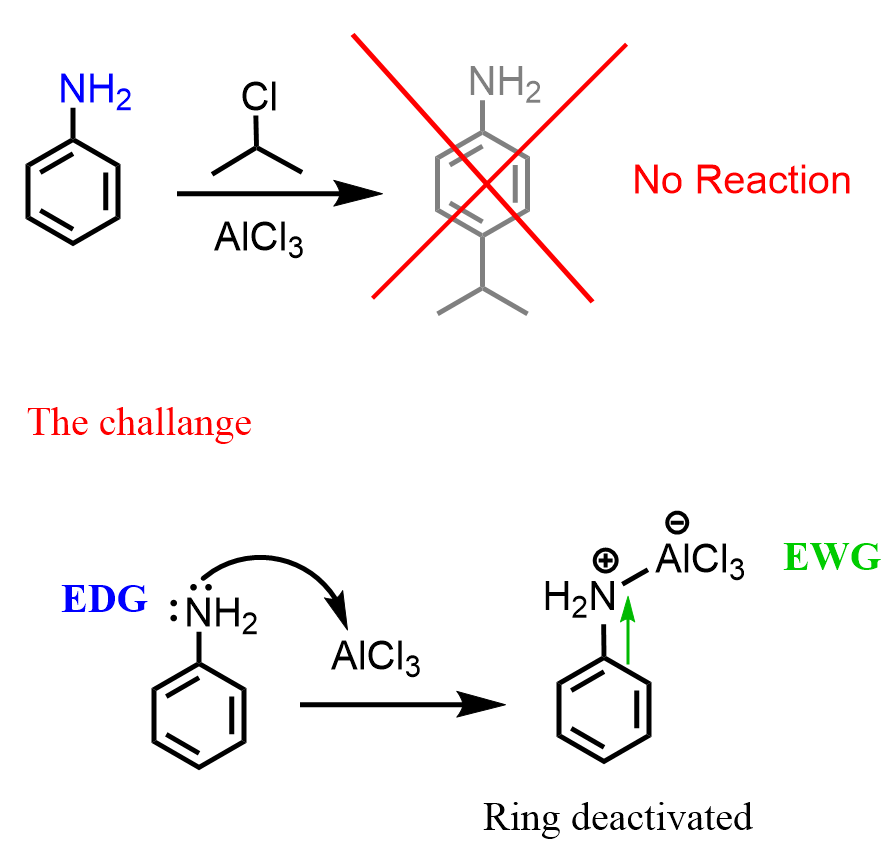
The positively charged nitrogen is no longer a resonance-activator because it pulls the electron density from the ring. Moreover, it is a strangely deactivating substituent on the ring, and Friedel-Crafts reactions, being slow, can no longer occur.
To overcome the problem with protonating the niteogen, thus turning it into a deactivator and a meta director, the same strategy of acylating the amino group is used:

Although the same problem exists for phenols, they can still be alkylated or acylated because the oxygen is not as strong of a Lewis base and does not bind to the Lewis acid catalyst to the same extent.
Nitration of Aniline
Even though the amino group activates the benzene ring, and some electrophilic aromatic substitution reactions of aniline are faster compared to those with benzene, nitration of aniline does not work as expected. We know that activating groups are ortho, para directors, so expecting ortho, para nitration of aniline might seem a reasonable prediction.
However, it has been shown that this reaction either leads to a mixture of undesirable side products, or, what is interesting, meta-nitroaniline is obtained:
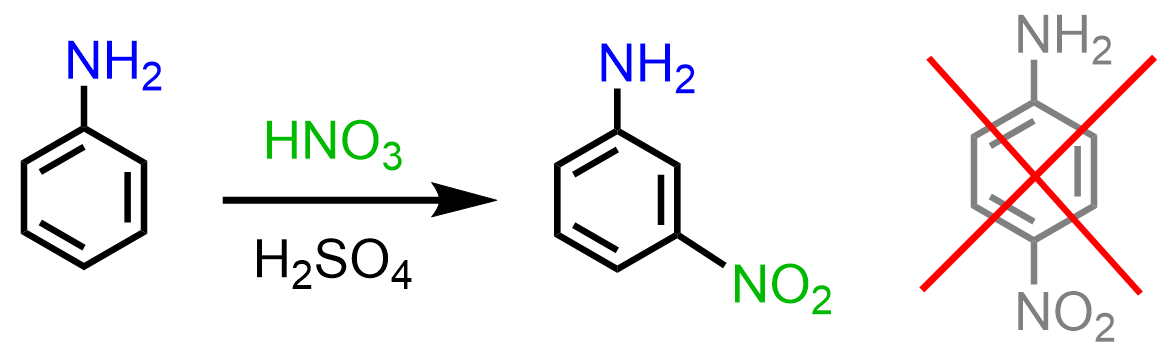
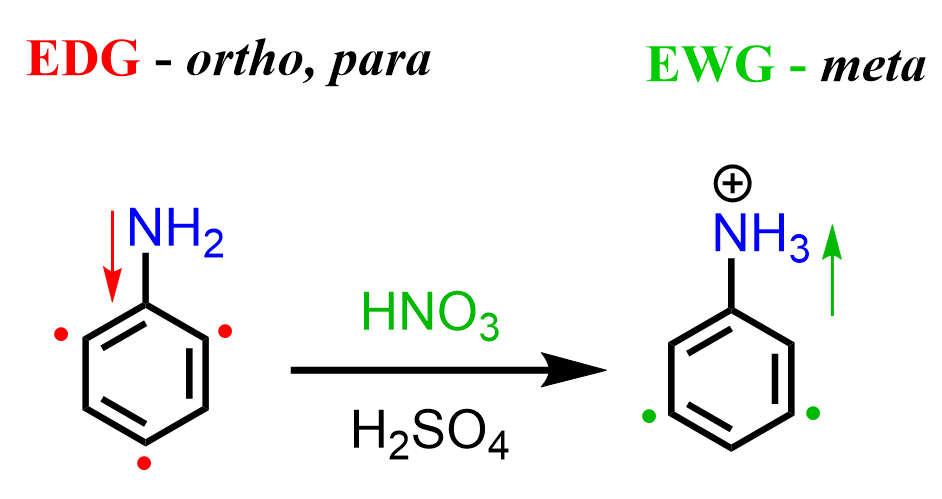
The formation of the unexpected meta product is again due to the basicity of the amino group. While in Friedel Crafts reactions, it acts as a Lewis base, here, we have a regular Bronsted acid-base reaction with HNO3 and H2SO4. Once the amino group is protonated, a strongly deactivating -NH3+ group is formed, which now undergoes a meta EAS:
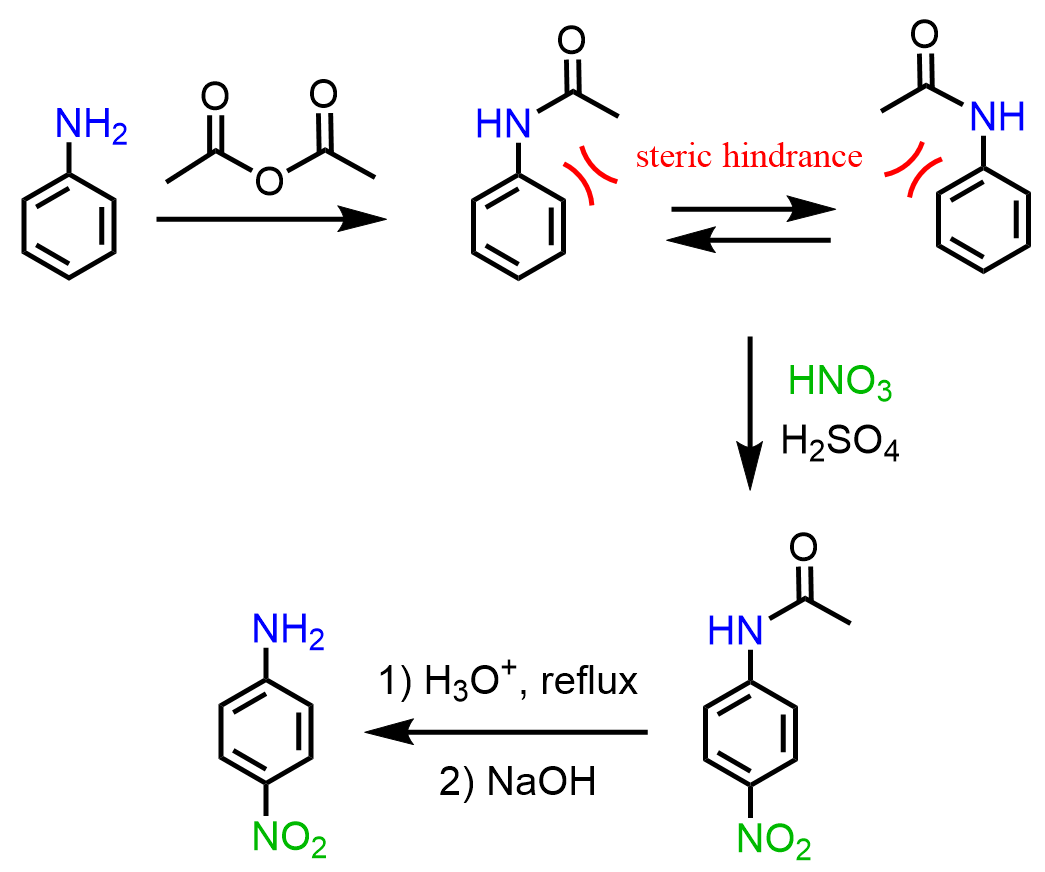
In this case, as well, the strategy is to convert the amino group to an amide and hydrolyze it back to amine (the other product is acetic acid) once the nitration is complete.
Are the EAS Actually Substitution Reactions?
In all the electrophilic aromatic substitution reactions, we have a substitution of a hydrogen by a new group. So, yes, these are substitution reactions; however, they are different from the ones we have seen in SN1 and SN2 reactions, where the leaving group was not a hydrogen.
Now, what if we want to replace, let’s say, a chlorine on a benzene ring with an OH or NH2 group?
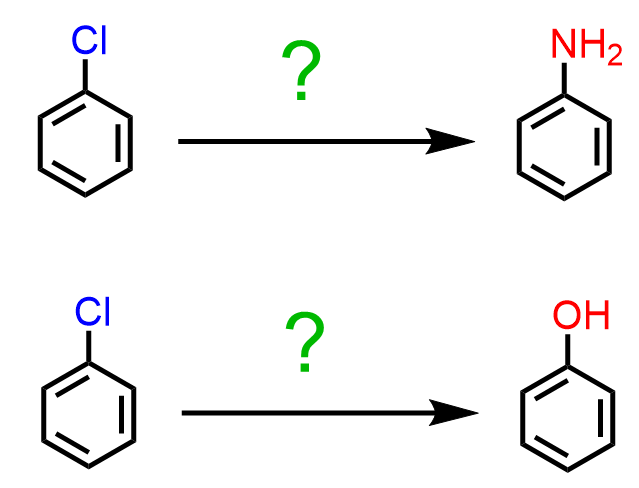
These types of conversions are achieved by nucleophilic aromatic substitution reactions. We have seen that most reactions of aromatic compounds involve electrophilic substitutions because the π electrons make the aromatic ring electron-rich and therefore, nucleophilic. However, some aryl halides with a strong electron-withdrawing substituent(s) on the ring can undergo nucleophilic substitution (SNAr) instead of electrophilic substitution:

X here is the leaving group, and the EWG stands for an electron-withdrawing group (most often nitro), which is there to activate the ring by making it electron-deficient.
There are two main mechanisms: addition-elimination or elimination-addition, also known as the benzyne mechanism, which is sometimes considered as a separate reaction. The benzyne mechanism works mainly with a very strong base and nucleophile, NH2–, and does not require an EWG group, although its presence is not a hurdle. Let’s put some common examples of these mechanisms and look into the details of the main patterns.

Check the linked article for more details about the mechanism and radiochemistry of these reactions.
![]()

