The benzylic position is quite reactive and presents a useful synthetic tool for preparing many aromatic compounds. The reason for this reactivity is the resonance stabilization of the benzylic carbon, regardless of whether the reaction goes through an ionic or radical mechanism. We will discuss each of these in the next sections.
Substitution and Elimination Reactions at the Benzylic Position
Going all the way back to the nucleophilic substitution and elimination reactions, we can recall that benzylic substrates readily undergo SN1, SN2, E1, and E2 reactions. For the SN2 and E2 mechanisms, it is simply a matter of having a strong base/nucleophile and a non-hindered carbon atom:

On the other hand, the reactivity in unimolecular SN1 and E1 reactions is explained by the resonance-stabilization of the benzylic carbocation formed in the rate-determining step:

Remember that carbocations are sp2-hybridized and the empty p orbital of the positively charged carbon is nicely aligned with the p orbitals of the aromatic system, which makes the cation resonance-stabilized:
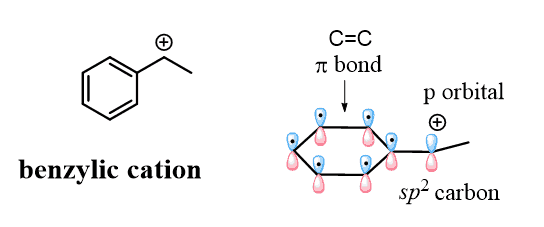
Don’t confuse the benzylic carbocation with the phenyl carbocation. Yes, they are both sp2 carbons, but unlike the benzylic carbon, the positive charge of the phenyl cation is a result of the empty sp2 orbital, which lies perpendicular to the conjugated aromatic system and cannot be resonance stabilized:
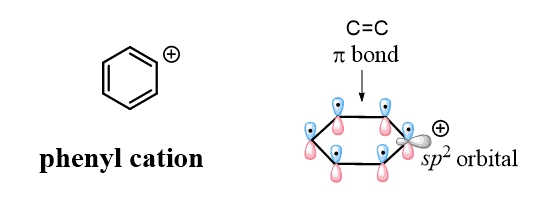
This is the reason, for example, why aryl and vinyl halides do undergo Fiedel-Crafts alkylation:

Halogenation of the Benzylic Position
Chlorination and bromination of the benzylic position are achieved when Lewis acid catalysts are not used:

The bromination can also be done using N-Bromosuccinimide (NBS), like in the allylic bromination:

All the halogenation reactions go by a radical mechanism, and the reactivity of the benzylic carbon is again explained by its resonance stability. Remember, radicals can also be resonance-stabilized, and that is how benzylic radicals are also quite stable.

Oxidation of the Benzylic Carbon
Strong oxidizing agents such as KMnO4 and Na2Cr2O7 oxidize a primary or secondary benzylic carbon to the carboxy group. The only requirement here is to have at least one hydrogen on the benzylic carbon. Therefore, the oxidation only works for primary and secondary alcohols:

Notice that even for longer alkyl groups, the remaining carbon atoms are cleaved off in the oxidation, and benzoic acids are formed.
A mild oxidizing agent can be used to oxidize benzylic alcohols to their corresponding ketones under carefully chosen conditions:

Reduction of the Benzylic Carbon
Aryl ketones prepared by the Friedel-Crafts acylation can be reduced to alkyl benzenes by the Clemenson or Wolff-Kishner reactions:

The mechanism and more details of these reactions are covered in this article.
Notice also that other ketones are reduced to alcohols, and only aryl ketones can be reduced to a methylene group by catalytic hydrogenation (H2 + Pt or Pd/C):

Reduction of the Nitro Group
Even though this is not a reaction at the benzylic position, I’d still like to add here the reduction of the nitro group on a benzene ring:

This reaction is important since there is no direct way of converting benzene into aniline, which is an essential precursor in the preparation of many aromatic compounds.
It is also used to prepare arenediazonium salts, which are again a great source for performing aromatic transformations.
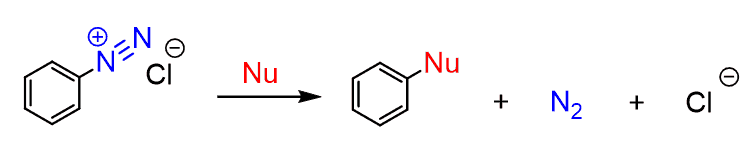
The Effect of the -COOH Group in EAS Reactions
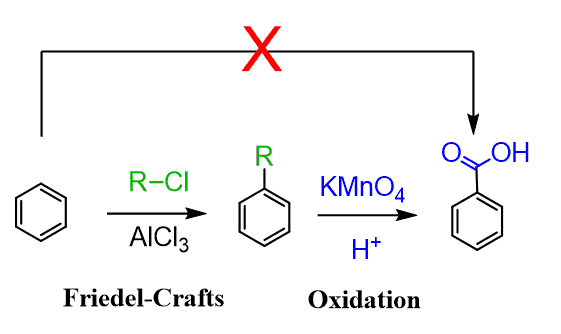
The benzylic oxidation is a great way to prepare substituted benzoic acids since the carboxy group cannot be added directly by electrophilic aromatic substitution.
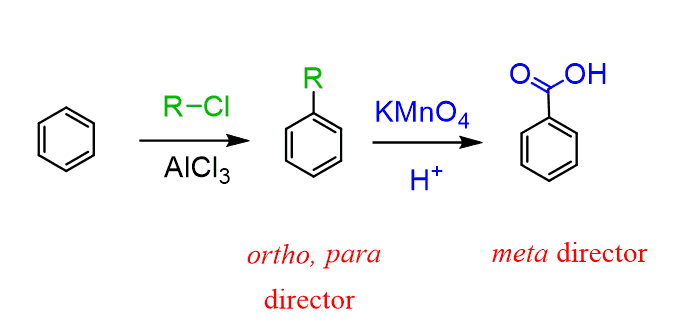
Once again, preparation of benzoic acids can be achieved through a Friedel-Crafts reaction followed by oxidation:
Another strategy for converting benzene or its certain derivatives to benzoic acids is the use of the Grignard reaction between phenylmagnesium halides and carbon dioxide. We can first halogenate the aromatic ring, add the Mg in dry conditions, and finally react it with carbon dioxide.”
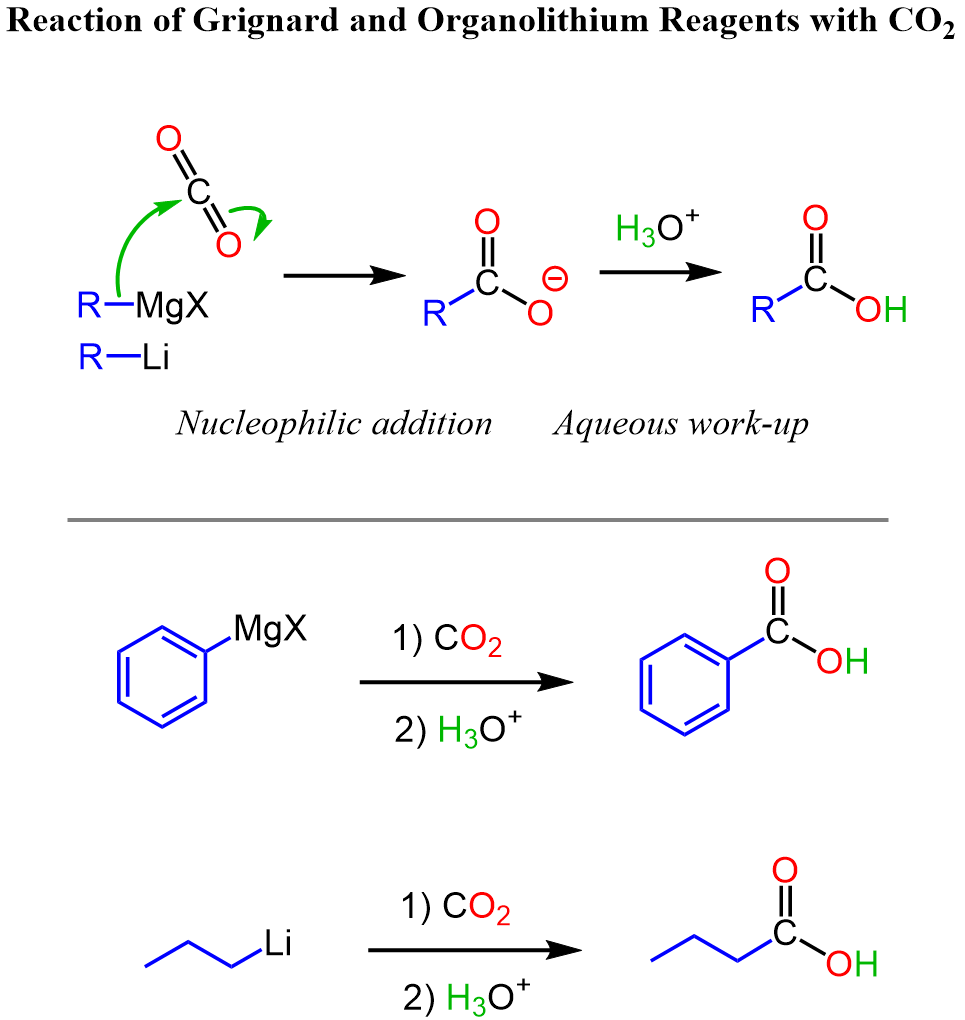
Going back to the method of alkylating benzene, then oxidizing the benzylic position, we should also emphasize the advantage of this method because alkyl groups are ortho, para–directing, while the carboxyl group is a deactivator and a meta director.
This allows us to synthesize some benzene derivatives that are not so trivial by the standard order of adding ortho, para-, or meta-directors
For example, how could you prepare p-Nitrobenzoic acid from benzene?
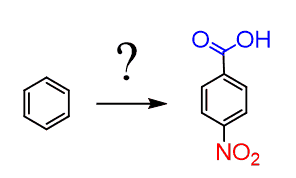
The problem here is that both groups are meta-directors, so there is no correct order of adding them to the benzene ring to have them in para orientation:
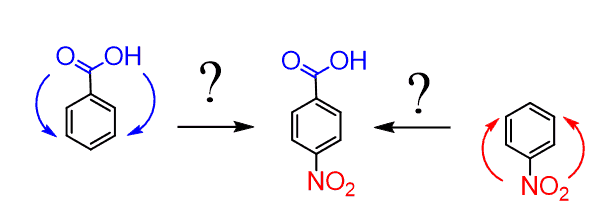
And this is where the method of oxidizing alkyl benzenes can be very useful. We can first add the methyl group by Friedel-Crafts alkylation, then nitrate the para position of the resulting p-Nitrotoluene, and only then oxidize the CH3 group to obtain p-Nitrobenzoic acid:

Notice that the reverse order would require a Friedel-Crafts alkylation of the nitrobenzene, but Friedel-Crafts reactions do not generally work with deactivated benzene rings. But even if we pretend they do, it still gives the undesired meta product:
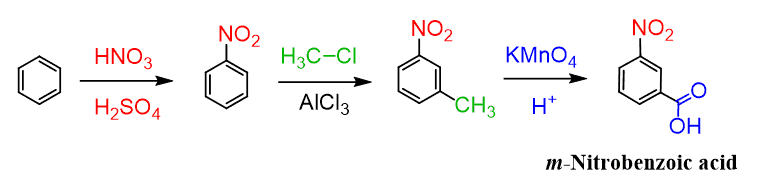
Ok, since we mentioned it: how do you prepare the meta-Nitrobenzoic acid then?
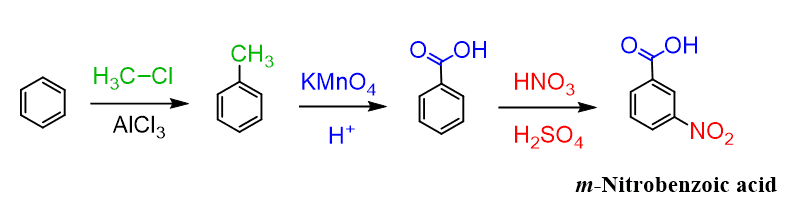
Since the methylation of nitrobenzene does not work, we need to go the other way, i.e., install the carboxy group first and then do the nitration, which will occur at the meta position since the carbonyl group is a meta director:
These are related to the synthesis of disubstituted benzenes, and if you need more practice problems on this, you can find them here:
Synthesis of Aromatic Compounds from Benzene
You can also try solving these multistep problems, which include a lot of aromatic chemistry. I will put one here, and you can check the rest on the linked article:
Organic Chemistry Multistep Synthesis Practice Problems
More on Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para– Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds

