We have seen earlier that under different conditions, alkanes, alkenes, and aromatic compounds react with bromine.
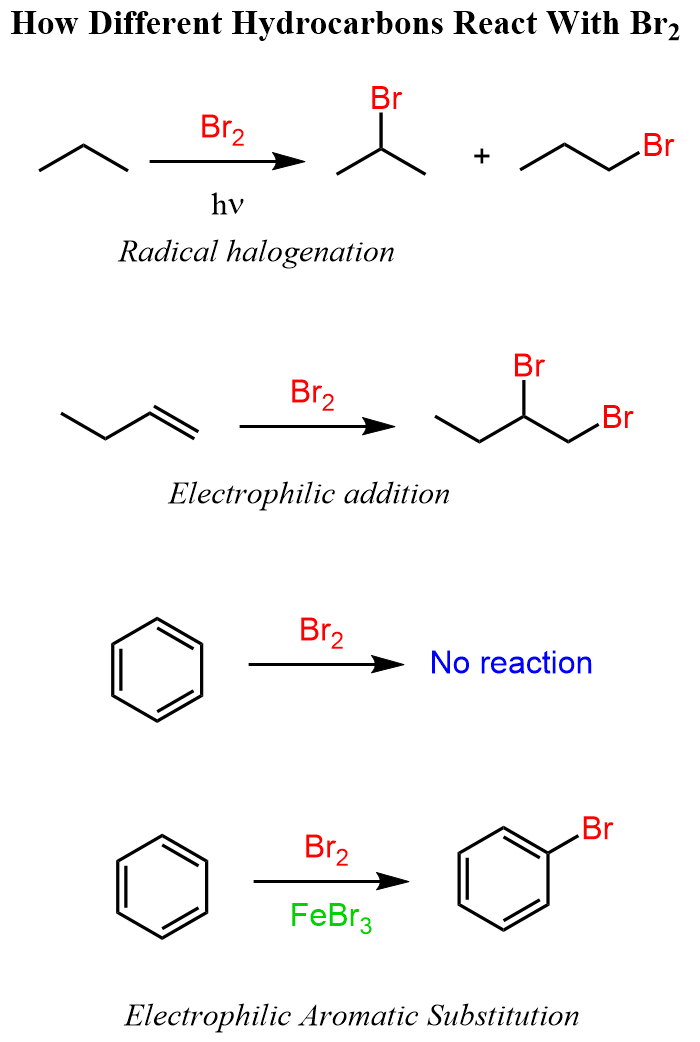
The halogenation of alkenes is explained by the electron-rich feature of the double bond, whereas aromatic compounds require a catalyst to make the bromine more electrophilic before they react with the π bond. This is explained by the stability of aromatic compounds, thus their unwillingness to break it for the sake of making a new C-Br bond. Each of these reactions is covered in a separate article, so feel free to check them for more details.
Recall also that the halogenation of alkanes proceeds via radical reaction and not ionic, such as the electrophilic addition to alkenes and electrophilic substitutions of aromatic compounds.
So, let’s consider a scenario where a molecule containing an aromatic ring and an alkyl chain is mixed with bromine. Where, if at all, would you expect the bromine to react?
The ring itself is quite stable and does not attack the bromine, so it is reasonable to expect a reaction on one of the saturated carbon atoms.
The carbon right next to the aromatic ring is the benzylic position, and it turns out that’s where the reaction mostly takes place.
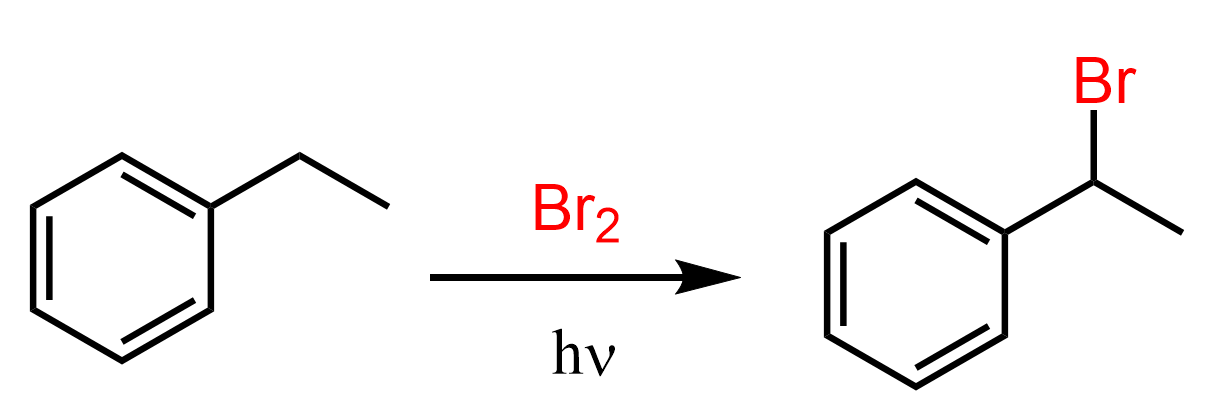
So, how do we explain this regioselectivity? One thing you may have noticed in your organic chemistry journey is that everything boils down to resonance structures. Well, not everything but a lot for sure. A more accurate and broader statement would be that everything occurs or at least is explained by two factors: sterics and electronics. To keep this short, sterics is the hindrance of the reacting center, thus affecting the outcome of the reaction (think of SN1 vs SN2), and electronics is essentially where the nucleophile or the Lewis base finds the electrophile or the Lewis acid.
Now, for today’s topic, we mentioned that the selective bromination of the benzylic position has to do with resonance structures, which fall into the category of electronics. What you need to remember is that, unlike the vinylic position, a charge or a radical in the benzylic position can be stabilized by delocalizing over the π system of the ring. Compare the orbital alignment in the phenyl and benzyl carbocations:
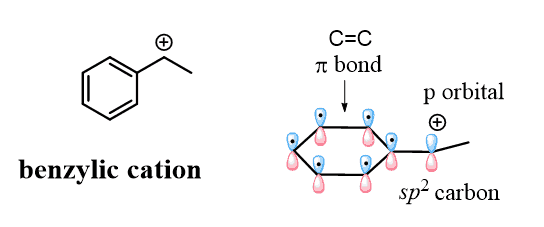
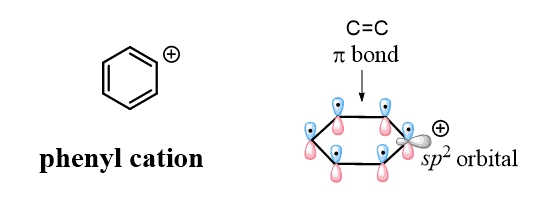
They are both sp2 carbons, but unlike the benzylic carbon, the positive charge of the phenyl cation is a result of the empty sp2 orbital, which lies perpendicular to the conjugated aromatic system and cannot be resonance stabilized.
And this is the reason why benzyl halides readily undergo unimolecular SN1 and E1 reactions, where the rate-determining step is the formation of resonance-stabilized benzylic carbocations:

You may not have covered this yet, but a good example demonstrating the instability of vinyl halides is that they do not undergo Friedel-Crafts alkylation because it requires the formation of corresponding carbocations:

Going back to the bromination of the benzylic position, you need to remember that radicals can also be stabilized by resonance. The mechanism is shown differently using one-headed (fishhook) arrows, but the concept is the same:
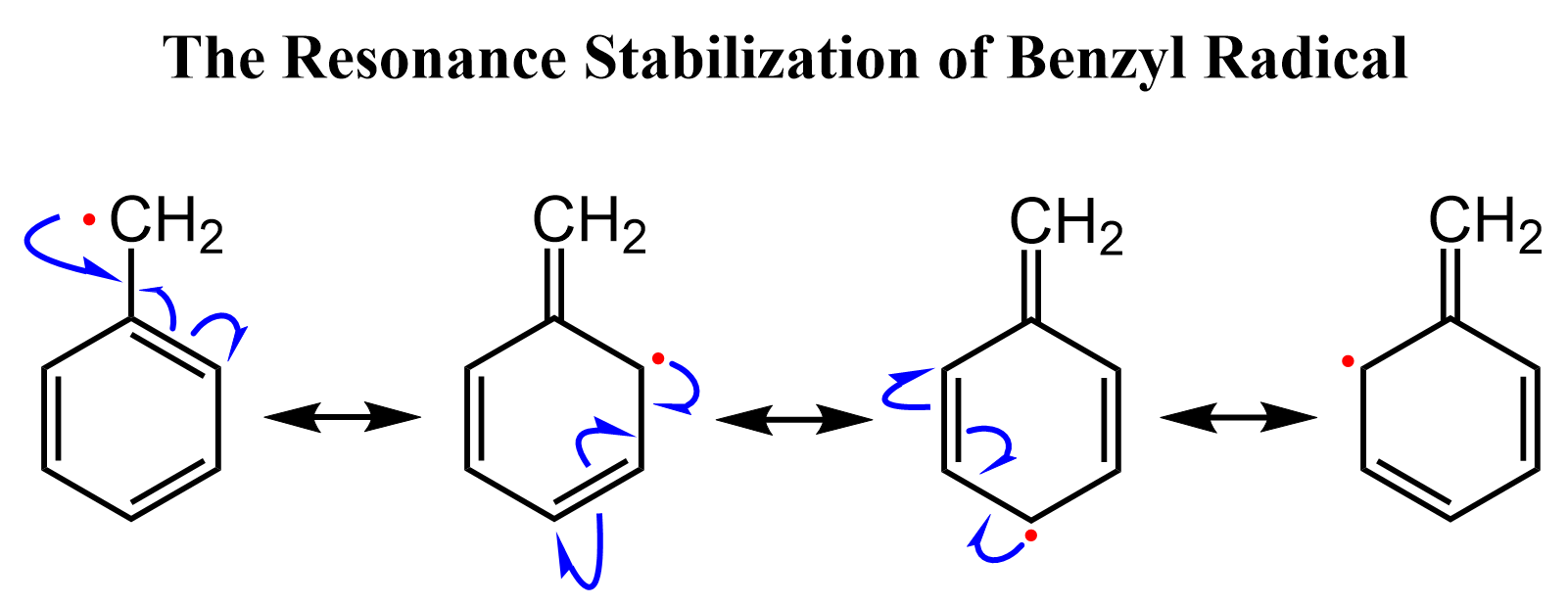
In general, radicals have the same pattern of stability as carbocations: electron-donation groups and resonance contributors increase their stability. So, the stability of radicals can be shown as follows:

Considering this stability pattern, we can see why the bromination occurs on the benzylic position. The radical that is formed is resonance stabilized and it is a secondary carbon while the CH3 is a primary carbon. Indeed, the bromination occurs exclusively in the benzylic position:
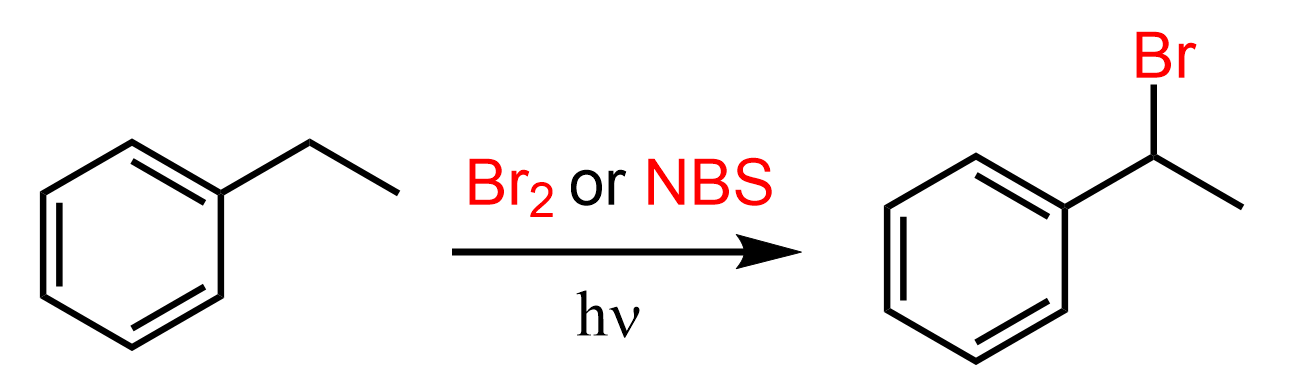
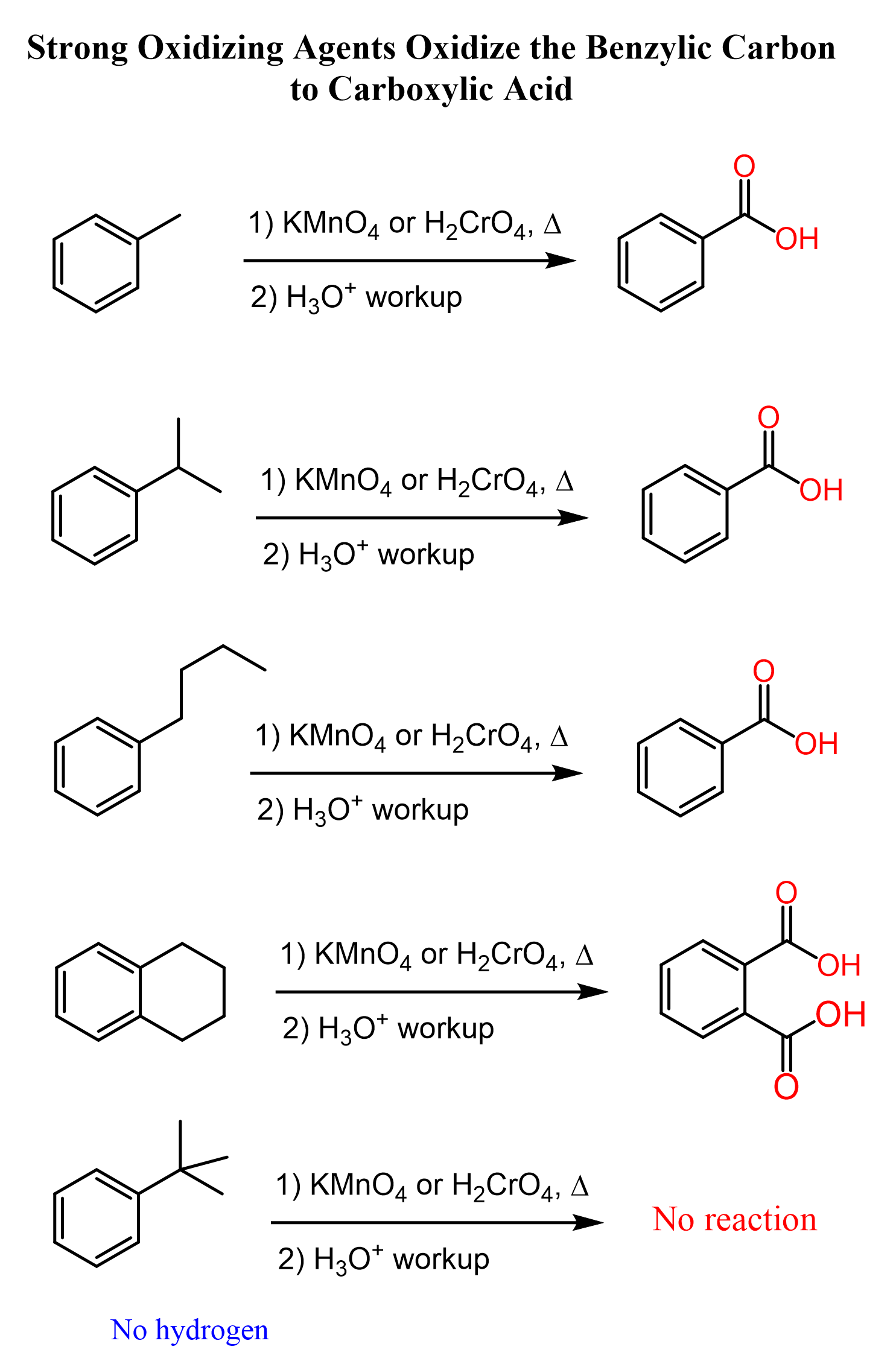
Now, if there were no hydrogens in the benzylic position, the bromination would still occur in the farther positions but it may not happen as fast.
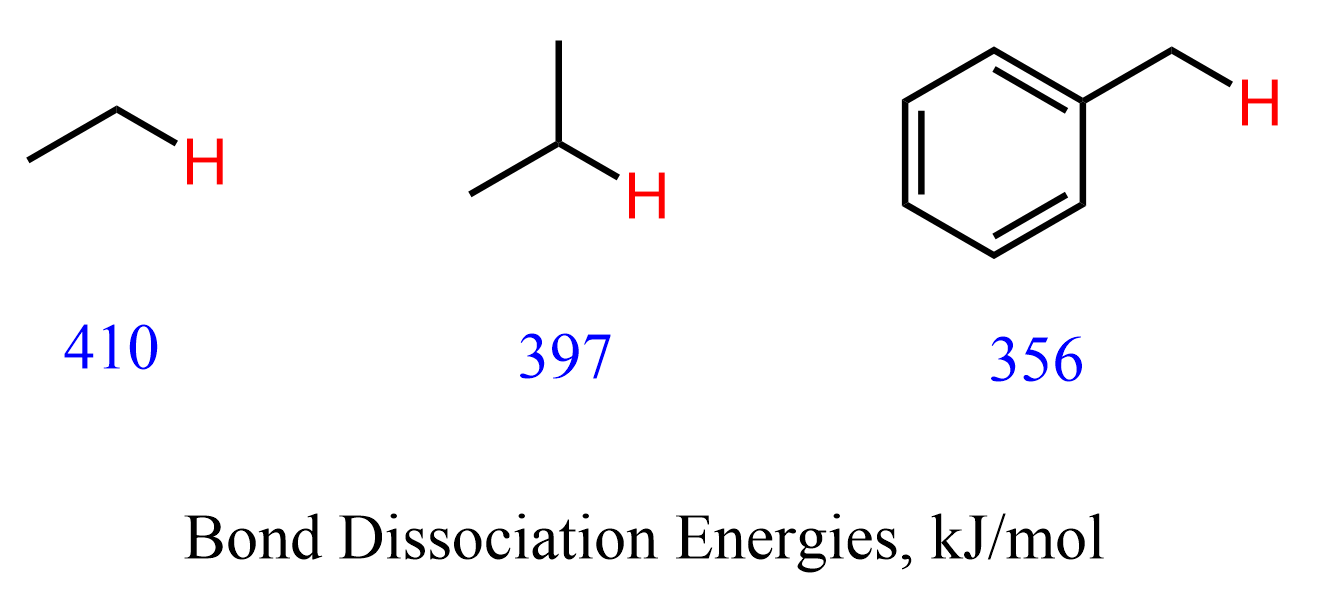
The selectivity of the benzylic bromination and chlorination can also be explained by the C-H bond strengths or the bond dissociation energies. Simple primary and secondary C-H bond dissociation energies are 410 kJ/mol and 397 kJ/mol, whereas a benzylic C-H bond requires only 356 kJ/mol energy for homolytic cleavage:
Let’s at this point show the complete mechanism for the bromination of the benzylic position by NBS, which is similar to what we saw in allylic bromination:
The Mechanism of Allylic Bromination
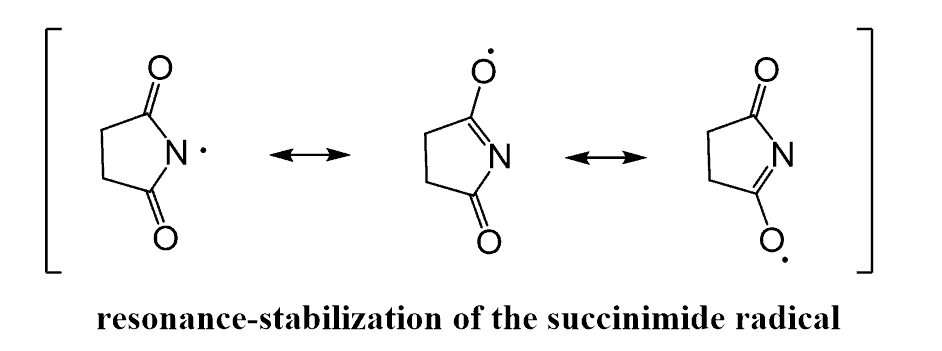
N-bromosuccinimide (NBS) is a common brominating agent, and its main advantage is that the bromine is produced in small quantities, preventing undesired side reactions.
The first step of benzylic bromination is the homolytic cleavage of the N-Br bond (initiation) of the NBS. This produces a succinic and bromine radical species. Once the bromine radical is formed, it abstracts a benzylic hydrogen, forming a resonance-stabilized benzylic radical (propagation), whereas the succinic radical reacts with the HBr, forming Br2 in small quantities, which is immediately attacked by the benzylic radical, giving the desired benzyl bromide.

The process repeats until the termination and consumption of the reactant(s).
Notice that the imide group can stabilize the radical by two additional resonance structures, which helps to initiate the homolysis of the N-Br bond:
The halogenation of the benzylic position is not exclusive to bromination. Chlorination can also be done:
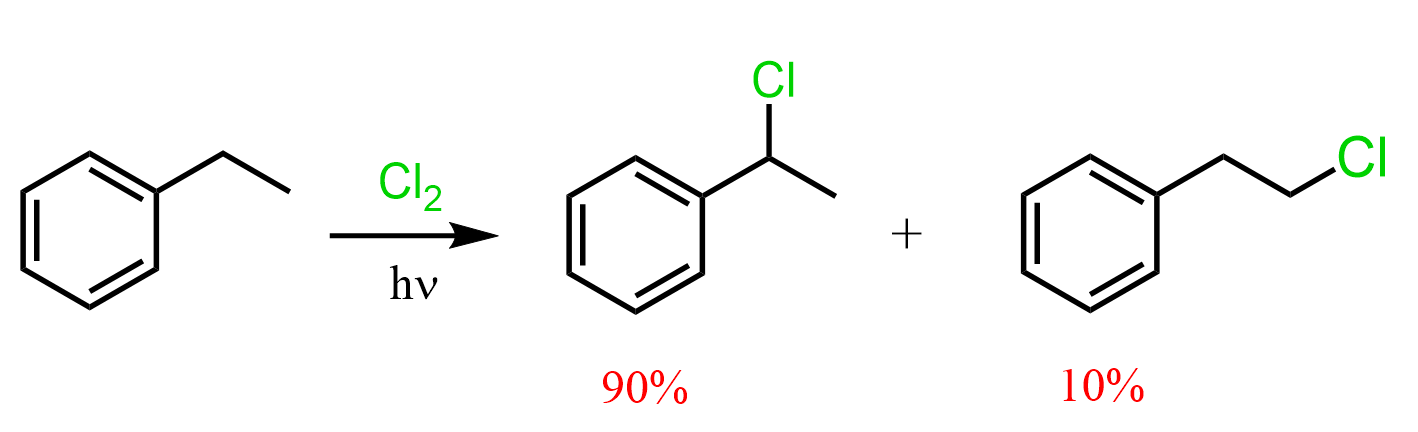
The selectivity is a little lower, and we have also seen this in the halogenation of simple alkanes:

The Applications of Benzylic Halogenation
Introducing a halogen into a carbon skeleton is perhaps the most common and important starting point for achieving a synthetic transformation. We have discussed this is a separate article about radical halogenation in organic synthesis. Simple alkanes contain only carbon and hydrogen atoms, and the absence of any imbalance in the electron distribution because of the lack of polar bonds makes them very much useless for functional group transformation.
Alkyl halides, on the other hand, can be used in many reactions, such as the simplest substitution and elimination reactions:
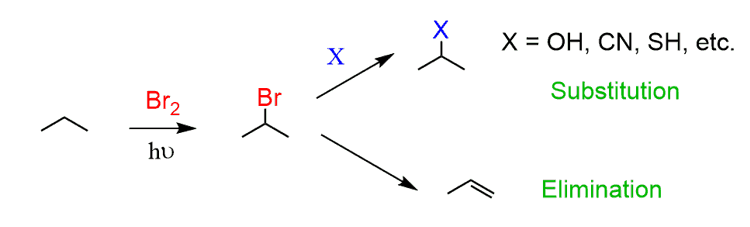
Now, aromatic compounds are not as inert as alkanes as we know (or will know) from many electrophilic substitution reactions, but halogenating the benzylic position plants a new seed to grow many other reactions. Some common ways of introducing functional groups are shown below:
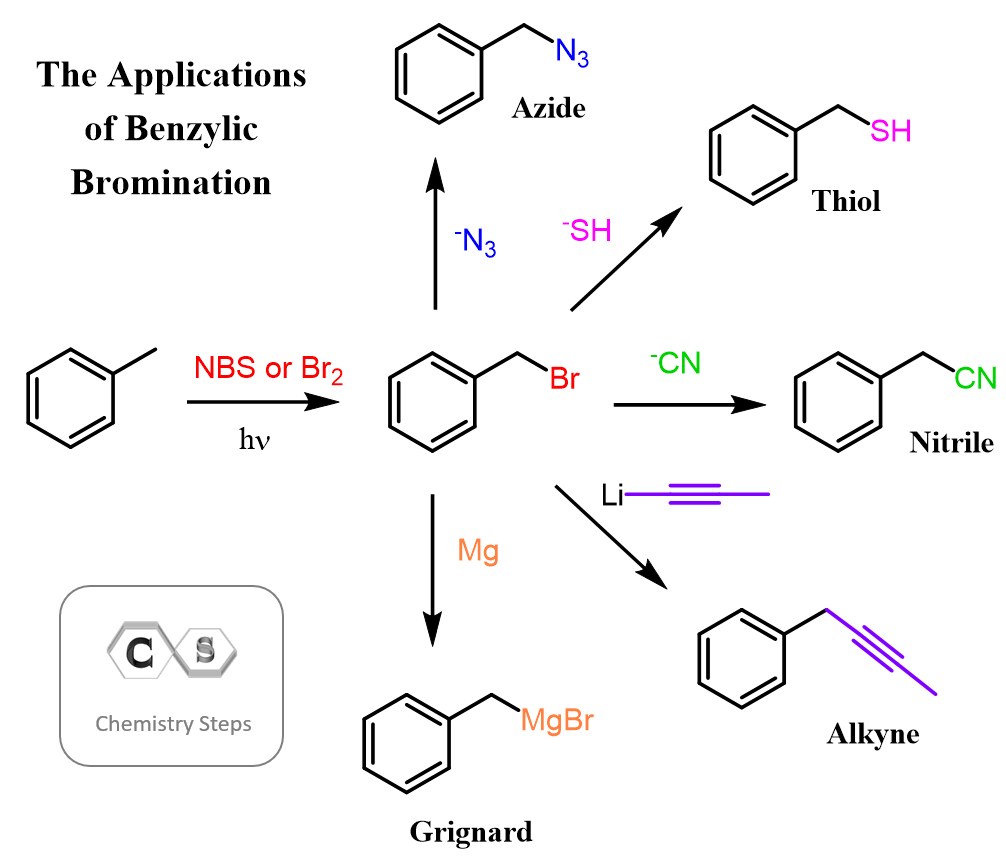
The new functional groups, in turn, can be used in different reactions. For example, the Grignard reagent is one of the most common organometallics you will see throughout your organic chemistry course.
Oxidation of the Benzylic Position
Aside from the halogenation, another way of functionalizing the benzylic position is the oxidation, which oxidizes the carbon to a carboxylic acid. The reaction is carried out using strong oxidizing agents, and what is important is that regardless of the chain length, the outcome is always a carboxylate group connected to the aromatic ring, as long as there is a hydrogen in the benzylic position:
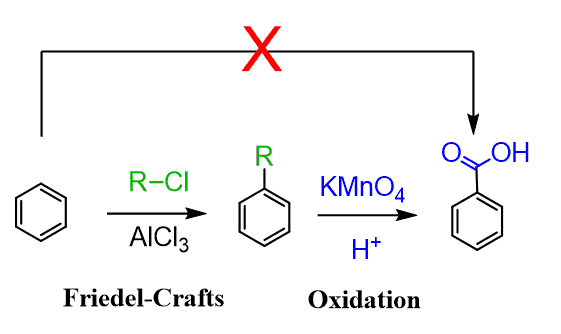
The benzylic oxidation is a great way to prepare substituted benzoic acids since the carboxy group cannot be added directly by electrophilic aromatic substitution, and it allows achieving it through a Friedel-Crafts reaction followed by oxidation:
The Carboxyl, COOH Group in Electrophilic Aromatic Substitutions
The benzylic oxidation is a great way to prepare substituted benzoic acids since the carboxy group cannot be added directly by electrophilic aromatic substitution.
Once again, preparation of benzoic acids can be achieved through a Friedel-Crafts reaction followed by oxidation:

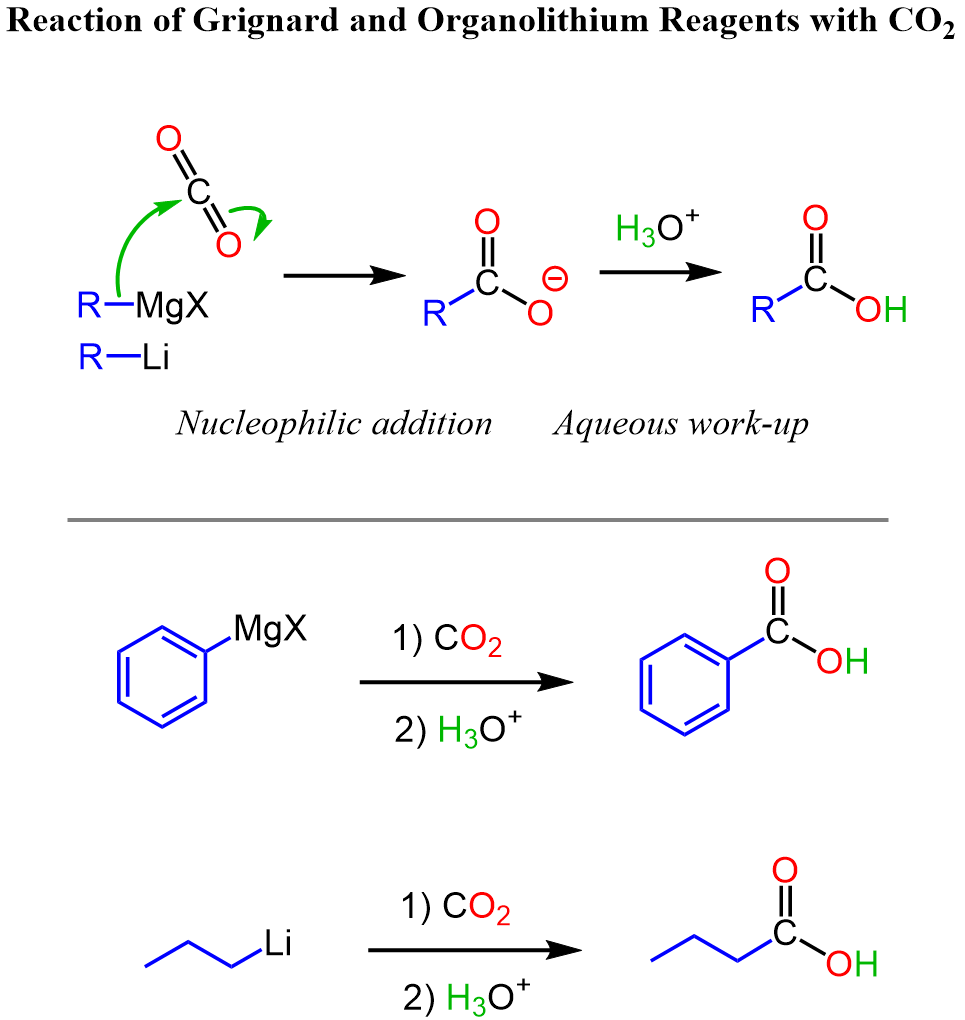
Another strategy for converting benzene or its certain derivatives to benzoic acids is the use of the Grignard reaction between phenylmagnesium halides and carbon dioxide. We can first halogenate the aromatic ring, add the Mg in dry conditions, and finally react it with carbon dioxide.”
Going back to the method of alkylating benzene, then oxidizing the benzylic position, we should also emphasize the advantage of this method because alkyl groups are ortho, para–directing, while the carboxyl group is a deactivator and a meta director.
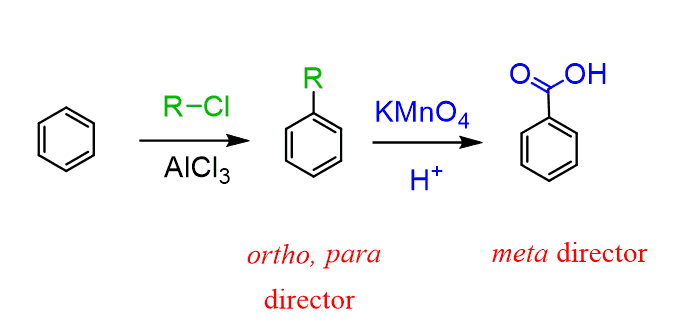
This allows us to synthesize some benzene derivatives that are not so trivial by the standard order of adding ortho, para-, or meta-directors
For example, how could you prepare p-Nitrobenzoic acid from benzene?
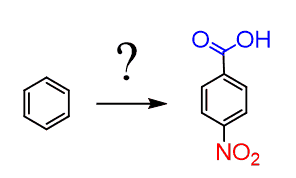
The problem here is that both groups are meta-directors, so there is no correct order of adding them to the benzene ring to have them in para orientation:
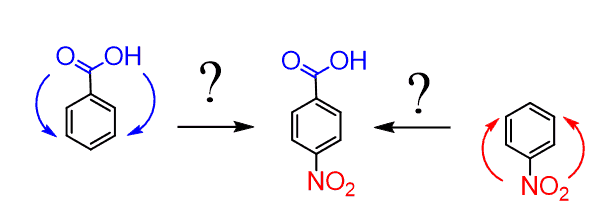
And this is where the method of oxidizing alkyl benzenes can be very useful. We can first add the methyl group by Friedel-Crafts alkylation, then nitrate the para position of the resulting p-Nitrotoluene, and only then oxidize the CH3 group to obtain p-Nitrobenzoic acid:

Notice that the reverse order would require a Friedel-Crafts alkylation of the nitrobenzene, but Friedel-Crafts reactions do not generally work with deactivated benzene rings. But even if we pretend they do, it still gives the undesired meta product:
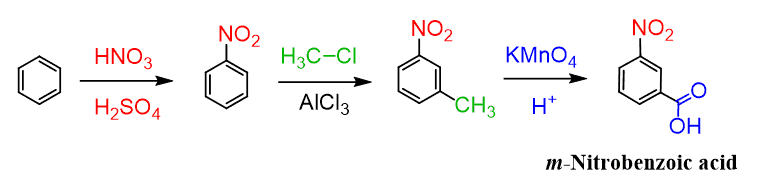
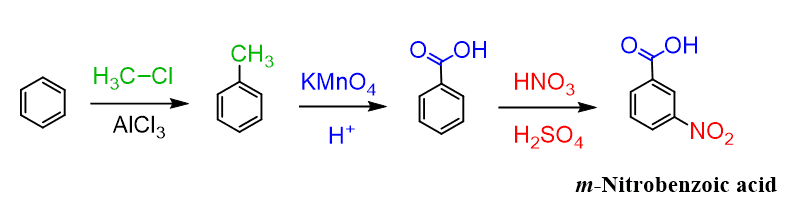
Ok, since we mentioned it: how do you prepare the meta-Nitrobenzoic acid then?
Since the methylation of nitrobenzene does not work, we need to go the other way, i.e., install the carboxy group first and then do the nitration, which will occur at the meta position since the carbonyl group is a meta director:
After the article, there are a few practice problems on synthesis that involve benzylic and allylic bromination as well. They may involve reactions you have not covered yet, but parts of them are doable with the knowledge of aromatic compounds.