Before talking about common exceptions in SN2 and SN1 reactions, let’s recall the main patterns we learned about them:
1) SN2 is favored for primary alkyl halides
2) SN1 is favored for tertiary alkyl halides
3) SN2 proceeds via inversion of configuration
4) SN1 reactions give racemization of chiral centers
Exceptions of Primary Halides in SN2 Reactions
We know that primary halides favor SN2 because they are sterically non-hindered, and the nucleophiles can easily access the carbon bearing the leaving group.
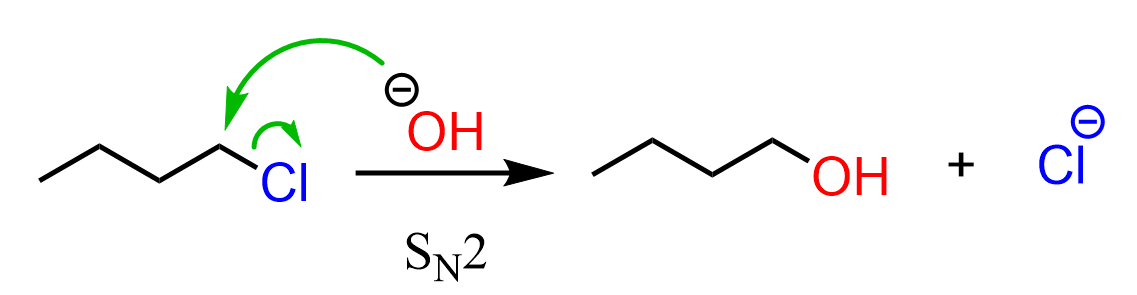
A common exception to this is the neopentyl halides, which practically do not undergo SN2 reactions, no matter what nucleophile we use:
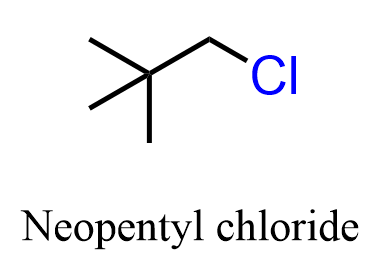
The reason for this is still the hindrance of the alpha carbon caused by alkyl groups not directly connected to it, but by those that are a carbon away.
The relative rates of SN2 reactions for different alkyl halides are given in the table shown below:

The Wedge and Dash in SN2 Reactions
The nucleophilic attack in SN2 reactions occurs from the opposite side of the leaving group; therefore, we know that if the leaving group is a wedge, then the nucleophile will be a dash in the product and vice versa:
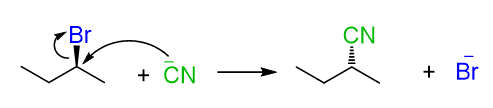
This is all true until we see a reaction when the leaving group is neither a wedge nor a dash. For example, which alkyl halide would give the desired product in the following SN2 reaction?
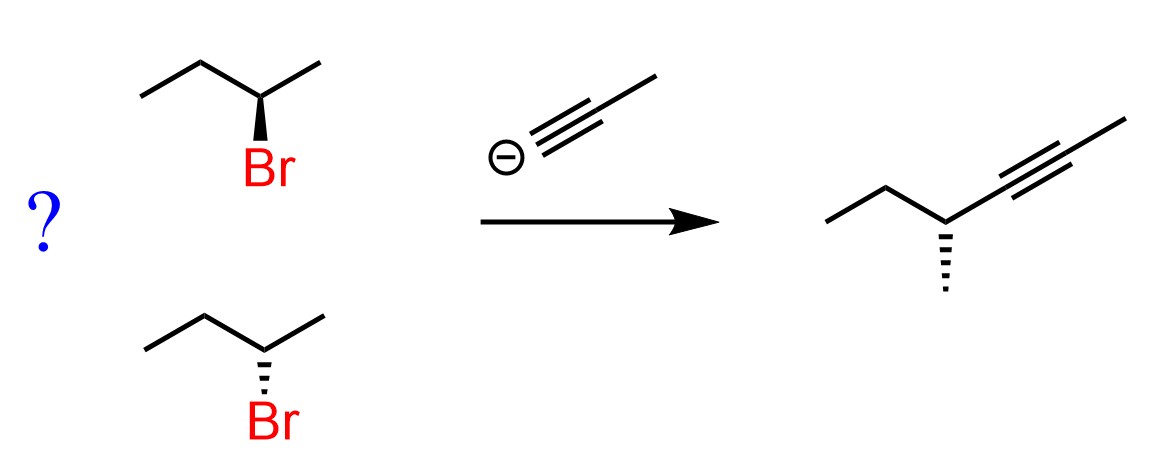
The first thing you may notice is that there is a dash bond in the product, so the leaving group in the reactant must be a wedge, and this would be the incorrect answer. The tricky part in these types of questions is that both the leaving group and nucleophile (in the product) are shown with the same plain lines. Normally, we see dash-wedge or wedge-dash conversion in SN2 reactions. However, remember that wedge and dash are relative representations, and if we rotate about an adjacent single bond, a dash may become a wedge and vice versa.
So, what you can do in these situations is keep the zig-zag as it is and draw the product like you would normally do. In this case, the nucleophile must be a wedge in the product:
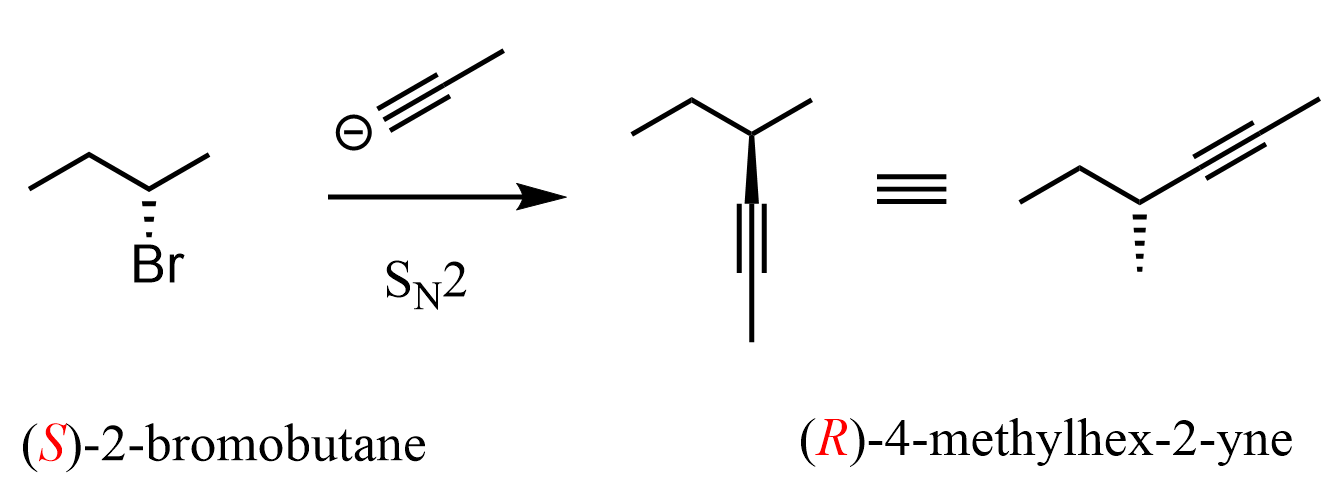
The product is simply another conformation of the one where the nucleophile is a wedge. If drawing different conformations is confusing, you can also go with the R and S configuration of the reactant and product. Remember, what we learned is that there is an inversion of configuration in SN2 reactions, so if the reactant is S, the product must be R, and if it is R, then the product must be S.
I want to mention that good nucleophiles that are also strong bases will mostly do E2 on secondary alkyl halides. However, I wanted to have a carbon nucleophile to address some tricky parts related to wedge and dash representations in SN2 reactions.
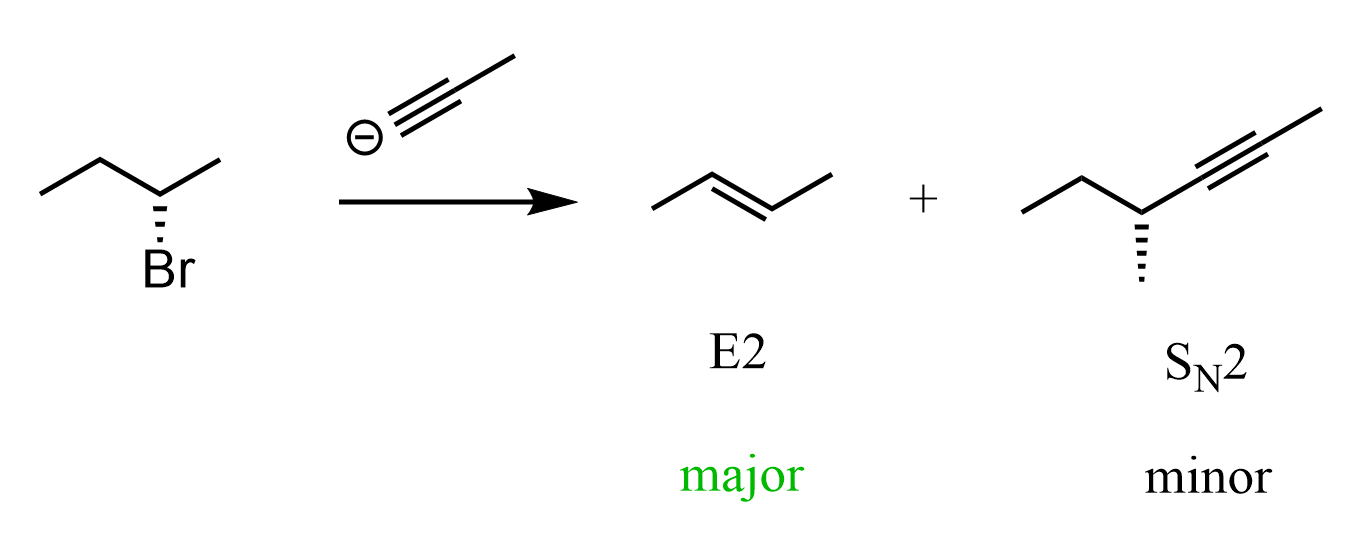
Another common confusion related to the wedge and representation of the leaving group and the nucleophilic in SN2 reactions is when the molecule is flipped in the product. For example, what is the mechanism and product of the following reaction?
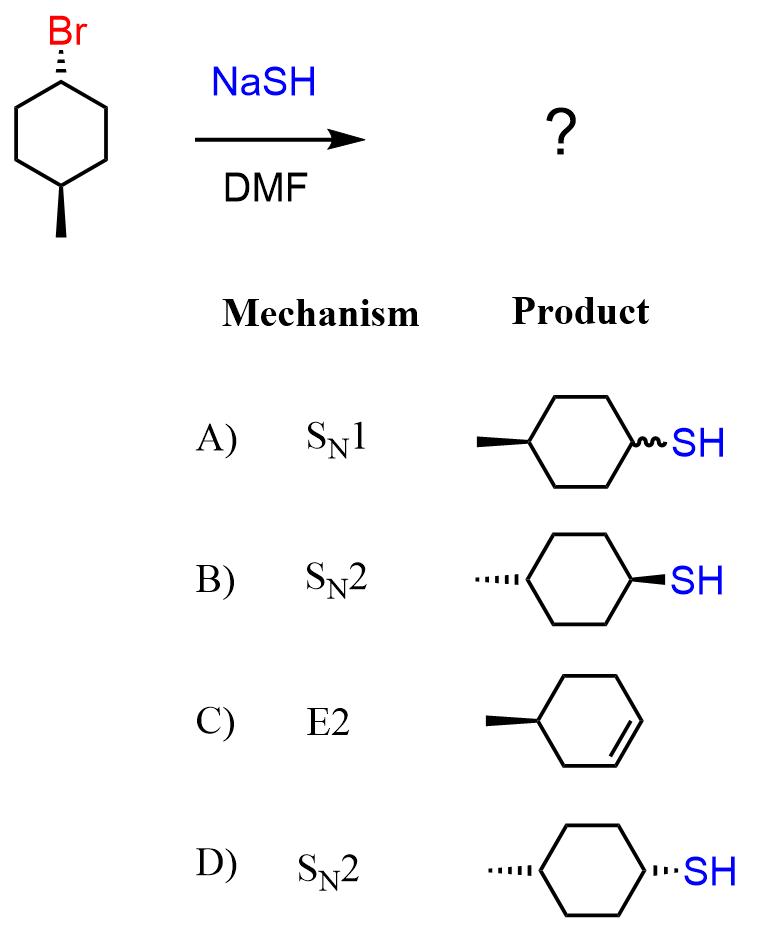
Seeing the leaving group as a dashed line, we automatically start looking for the product with a wedge nucleophile, and wrongly pick answer B. It is wrong because we need to pay attention to the methyl group and whether it is cis or trans to the leaving group. In this case, it is trans, which means that in the product, it must be cis to the nucleophilic. In the world of wedge and dash, we’d say the product must be wedge-wedge.
There is no wedge-wedge answer, but keep in mind that we can flip the molecule 180o and wedge-wedge becomes a dash-dash, which means answer D is correct:
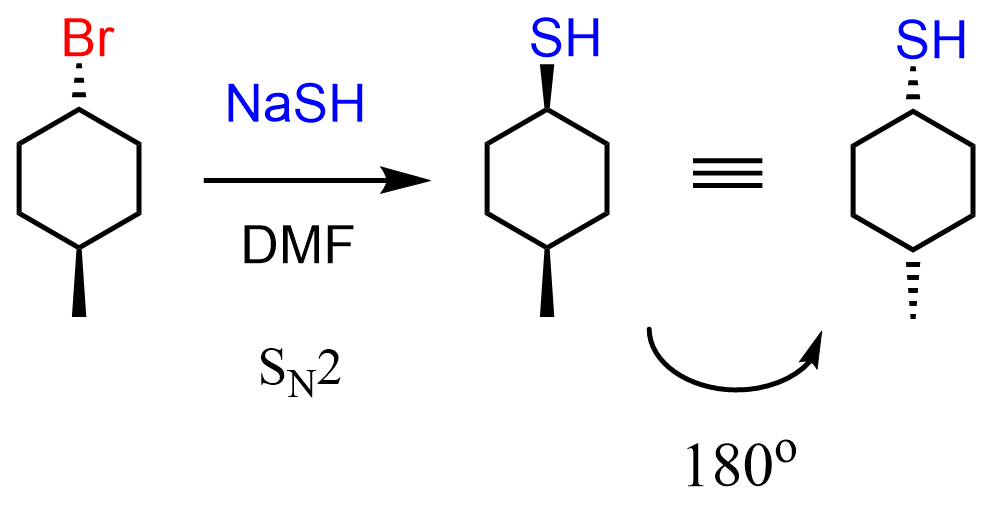
As to why the reaction is SN2 and not SN1, E1, or E2, there is also a separate post dedicated to deciding the correct mechanism, which you can find here. In short, it is because we have a strong, non-basic nucleophile which favors the SN2 mechanism over the E2 elimination. These two mechanisms are also compared in this post.
🟢 CS members, don’t forget to check out the Reaction Map of Alkyl Halides – all the key reactions, and nuances in determining the correct mechanism are summarized there in one place for quick review and reference!
R and S Configuration is SN2 Reactions
What is important to remember about SN2 reactions is that the nucleophilic attack occurs from the opposite side of the leaving group. In most reactions, since the leaving group is a halogen and the nucleophile is a heteroatom (O, N, S, etc.), they are both of the highest priority when determining the R and S configuration. This leads to the R-S and S–R inversion.
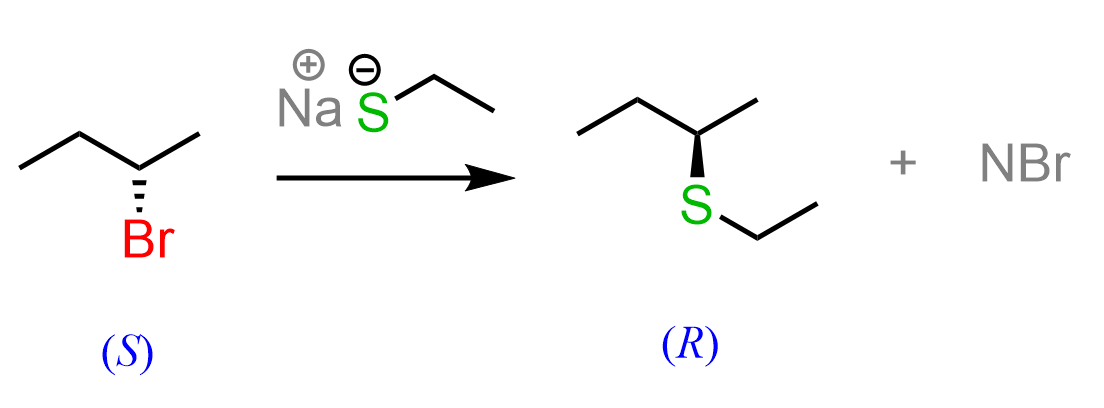
If an “unusual” nucleophile or a substrate is used, we may see R–R or S–S retention in configuration, while in reality, the physical configuration of the chiral center is in fact, inverted:

Retention of Configuration in SN2 Reactions
The example we discussed above is rare, but what if you are given a question where the leaving group and the nucleophile are both wedge or dash? You check the R and S configuration, and in fact, yes, there is no inversion of configuration:
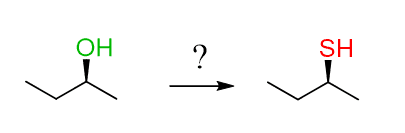
Most often, this should hint to you that there are two consecutive SN2 substitutions on the substrate. The starting material here is S, and an SN2 reaction would change it to R. However, if we do two SN2 reactions it will go S-R-S:

One approach for achieving this would be converting the OH into a halide, followed by a reaction with sodium sulfide. There are different ways of converting an alcohol into a good leaving group, and you may not be familiar with some of them now:

The method shown above uses the mesylation of alcohols to convert them into a good leaving group, which can then be expelled by the Br– and undergoes another SN2 reaction with the sulfide ion.
This was a three-step synthesis applying double SN2 to retain the absolute configuration, but:
Tertiary Alkyl Halides that Do Not Undergo SN1
We know tertiary alkyl halides do not undergo SN2 substitutions because the carbon with the leaving group is sterically hindered. Instead, they undergo SN1 substitution because the carbocation intermediate is fairly stable due to the electron-donating effects of the connected alkyl groups:

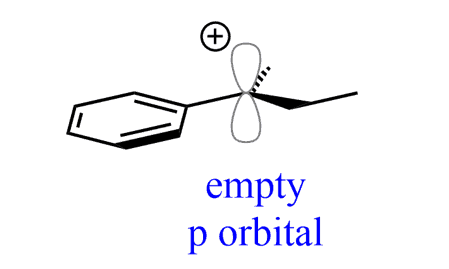
This stabilization of the carbocation occurs via the donation of electron density from the bonding orbitals of the neighboring C-H bond to the empty p orbital of the positively charged carbon atom. This is called hyperconjugation:

Keeping this in mind, why do you think the following bicyclic alkyl halide does not undergo an SN1 substitution (neither does it SN2)?
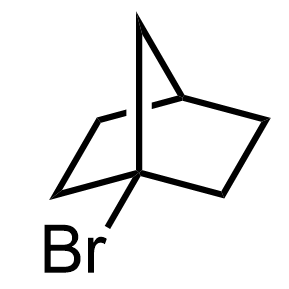
What you need to know is that electron donation via hyperconjugation occurs only if the sp2 orbital of the carbocation is parallel with the electron-donating sigma bond(s). In this example, the loss of the leaving group is very unfavorable because the carbon connected to the halogen is locked in a tetrahedral geometry. This means that it cannot be planar and thus stabilized by neighboring alkyl groups:
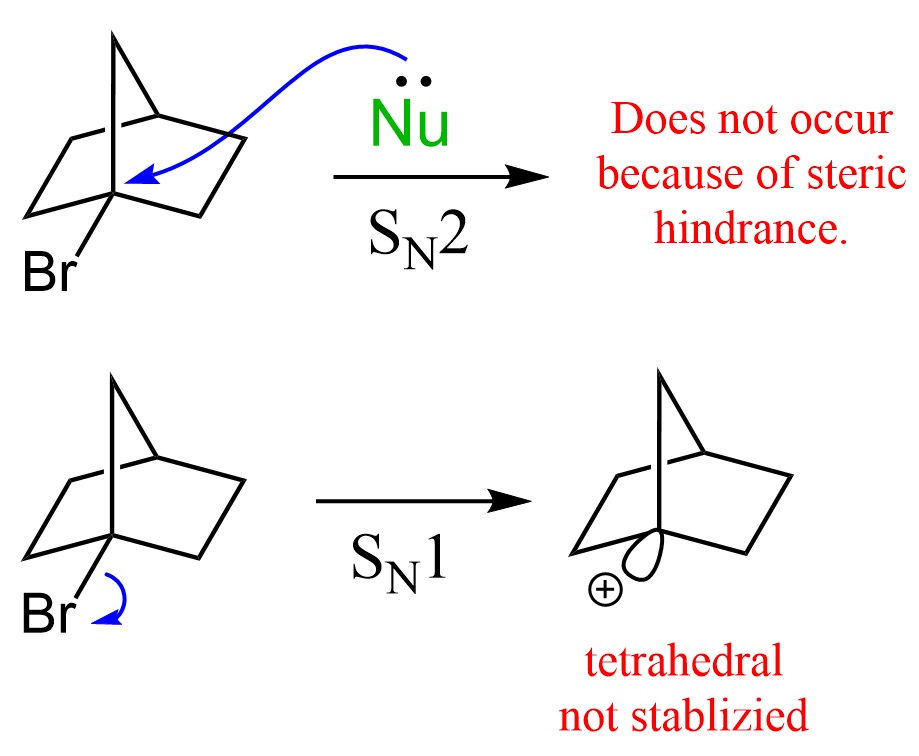
The Stereochemistry of SN1 Reactions
We know that SN1 reactions proceed via the formation of carbocation intermediates, and the nucleophilic attack on them occurs from both faces of the planar carbocation. As a result, a pair of enantiomers is formed when the addition of the nucleophile generates a chirality center:
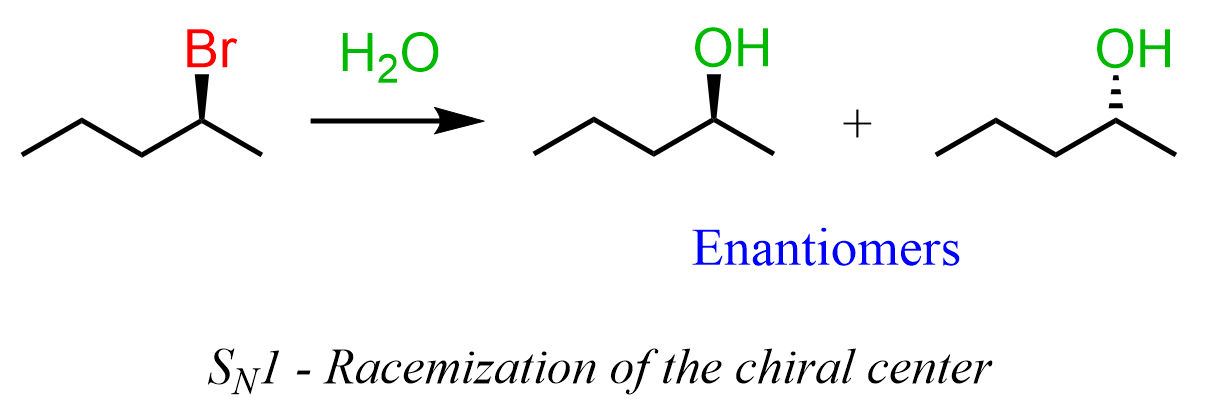
All true, but sometimes you might be given a substrate that already contains a chirality center(s). For example, what is the relationship of the products formed in the following substitution reaction?
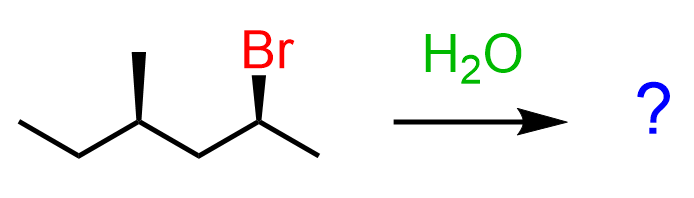
If this chirality center is not part of the substitution, and a new chirality center is formed, the product is going to be a mixture of diastereomers:
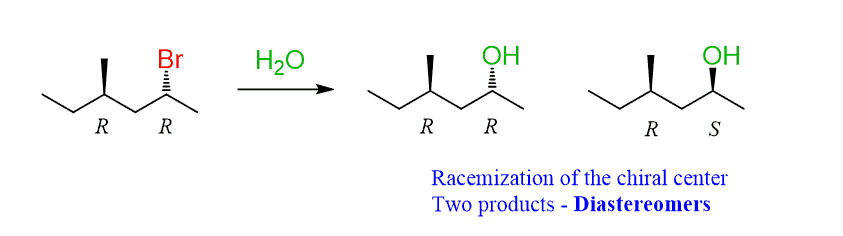
While the configuration of the original chirality centers is intact, the new one is forming as a mixture of R and S configurations, and therefore, these isomers are diastereomers.
Rearrangements in Substitution Reactions
Do not automatically replace the leaving group with the nucleophile in subsection reactions. Always keep in mind the possibility of rearrangements when you see tertiary and secondary carbons next to each other. For example, what is the mechanism and major product of the following substitution reaction?
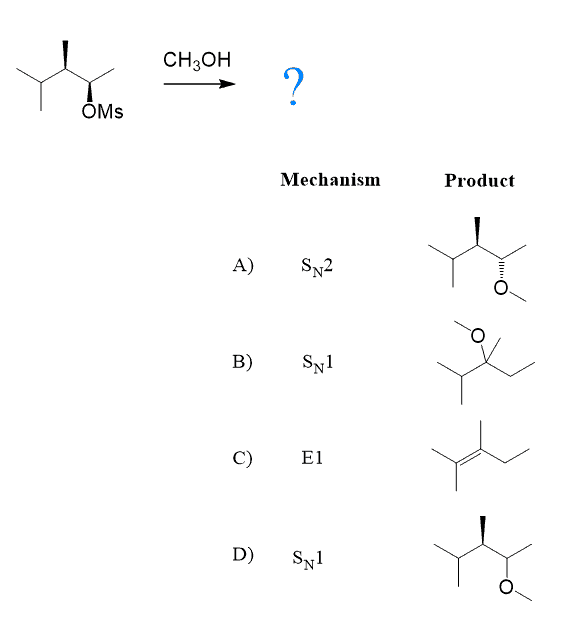
The first thing here is to realize that we have a weak nucleophile; therefore, the reaction must be SN1 or E1, and since heat is not mentioned, it is mostly going to be SN1.
If it is SN1, always draw or visualize the carbocation that would be formed after detaching the leaving group:
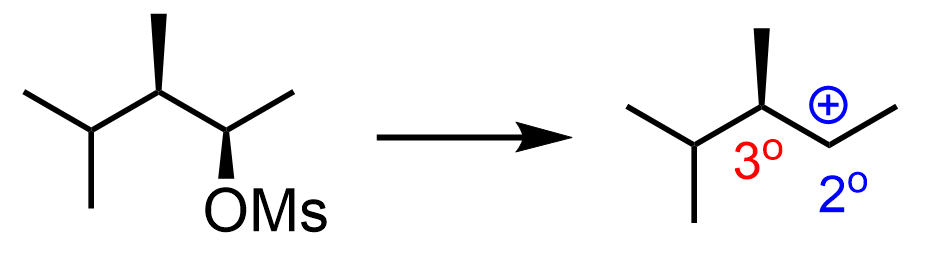
It is a secondary carbocation next to a tertiary carbon, so it is going to be rearranged into a more stable tertiary carbocation. The nucleophilic attack of the water will form a racemic mixture of enantiomers:
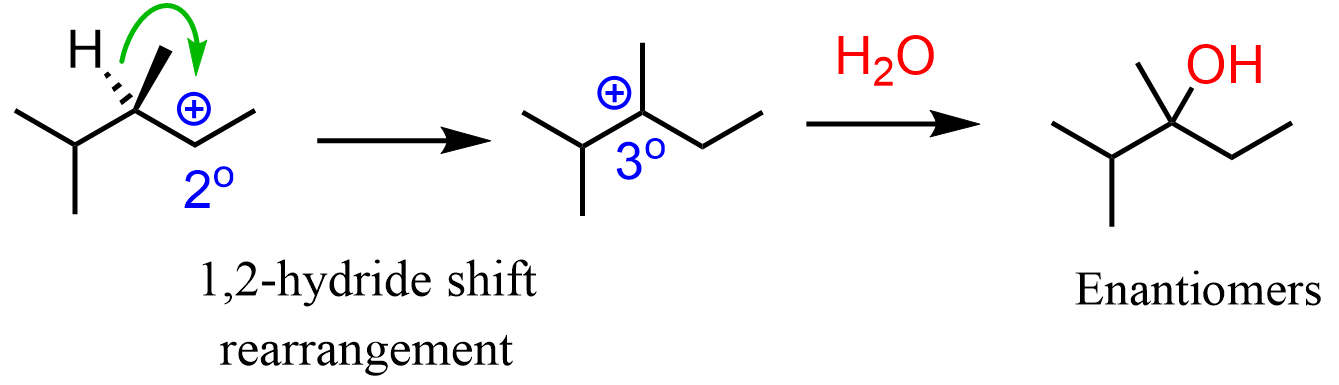
Notice that the chirality centers are shown with plain lines, which means none of the enantiomers (wedge and dash) is formed exclusively or in excess.
If you need to practice, there are more of these types of questions in the multiple-choice quiz on substitution and elimination reactions.
Bulky Reactants Doing SN2
You may have already talked about the competition between SN2 and E2 reactions in class. These are favored with strong/good nucleophiles and bases, and the outcome of the reaction is often determined by the steric hindrance factor. In short, steric hindrance suppresses the reactivity in the SN2 mechanism, and if a strong base is used, the E2 elimination predominates.
For example, 1-bromopropane is a primary alkyl halide, and it gives an SN2 product when reacted with an ethoxide ion (non-hindered strong base/nucleophile), whereas the reaction with Potassium tert-butoxide, tBuOK, a sterically hindered strong base, gives E2 elimination.
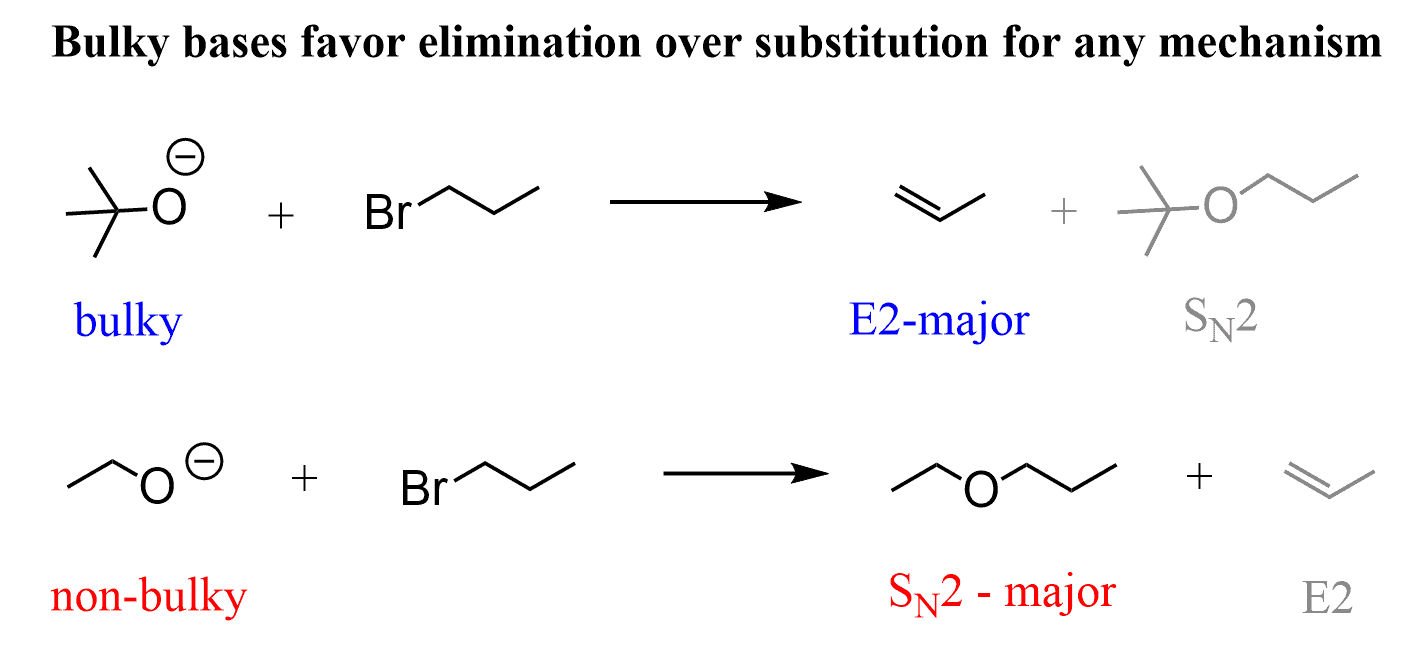
So, any alkyl halide that has a β-hydrogen will undergo an E2 elimination when a strong, bulky base such as t-BuOK is used. One exception that comes to mind is the use of the least sterically hindered alkyl halide. That doesn’t sound right, does it?
The thing is, we are talking about methyl halides, and there are simply not enough carbon atoms to make a double bond; therefore, the outcome of this reaction is an SN2 substitution:
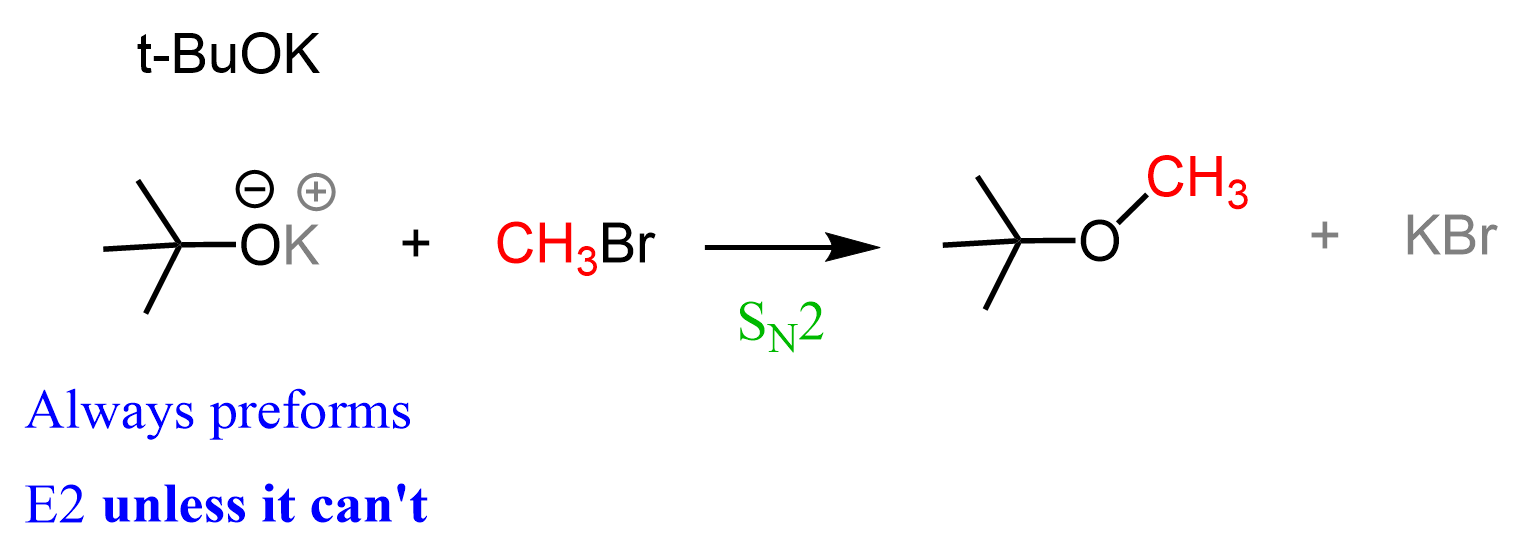
You can read more about the comparison and competition of SN2 and E2 reactions here.
These are the most common examples of exceptions in substitution reactions that will be helpful to know for your test. Let me know in the comments if you have questions or if there are other exceptions you’d like to see here.
Check Also
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- Steric Hindrance in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
