What are the Fischer Projections?
Fischer projections are just another way of drawing compounds contacting chirality centers. They were initially proposed by Emil Fischer for making it easier to draw the structures of compounds containing multiple chirality centers, with the main idea of not having to draw the wedge and dash lines for every single chiral center. This is especially applicable and used mostly for drawing sugars.
How to draw Fischer projections
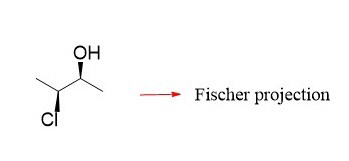
Suppose you have this compound with one chirality center:

Before getting to drawing its Fischer projection, let’s number the carbons in any order (no IUPAC rules needed). Remember, numbering carbons will always be helpful, no matter what you need to do with an organic structure.

Here is what Fischer suggested:
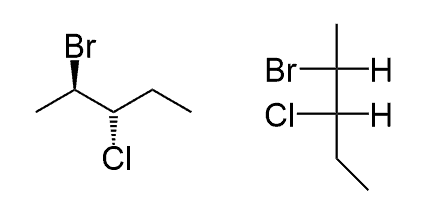
If you look at the molecule from the top, you will see the following representation where the two groups on the side are pointing towards you and the ones on the top and the bottom are pointing away from you. We will show the ones on the sides with wedge lines and the others with dashed lines:

There are two wedge and two dash lines, which may look strange to you since we always have one of each, and then the two solid lines, but it is okay-it all depends on the direction we are looking at the molecule.
This, however, is not the Fischer projection yet, since, remember, we said the main idea was to avoid showing wedge and dash lines, yet being able to convey the absolute configuration of the chirality centers (R, S).
For this, we are going to draw the molecule and simply show all the bonds with plane solid lines, keeping in mind that the horizontal groups are pointing towards you and the ones on the vertical line are pointing away from you:

So, how do you remember which ones are pointing towards you?
Well, you can remember that Fischer projections like you and they are coming to give you a hug with open arms:

Or, you look at the Fischer projection like you are in the gym and need to grab the molecule. In this case, as well, the horizontal groups have to be pointing towards you.

Determining the absolute configuration (R, S) of Fischer projections
To determine the absolute configuration of chirality centers in a Fischer projection, we need to follow the same steps as we do for any other representation, such as Bond-line or Newman, according to the Cahn-Ingold-Prelog rules.
In this molecule, for example, we need to assign the priorities of the groups on the chiral center based on the atomic numbers:

Next, draw the arrow going from priority 1-2-3:

The arrow goes clockwise, which indicates R configuration. However, this is where you need to remember that the horizontal groups (Cl and H) are pointing towards you; therefore, the configuration must be switched from R to S. This is because one of the rules of the Cahn-Ingold-Prelog system is that the lowest priority must point away from the viewer.
How to draw the enantiomer and a diastereomer of a Fischer projection
Fischer projections make it easy to draw different stereoisomers. As an example, if you are asked to draw the enantiomer of the following molecule with three chiral centers, you can draw an imaginary mirror plane and draw the reflection of the molecule, which is achieved simply by swapping the two groups of a chiral center:

To confirm that these two are enantiomers, assign the absolute configuration of the chirality centers. They are all inverted from R to S and S to R.
If you need to draw a diastereomer of the molecule, you need to switch only one chilaity center or, alternatively, switch all except one. In other words, the two representations should be non-mirror-image stereoisomers:

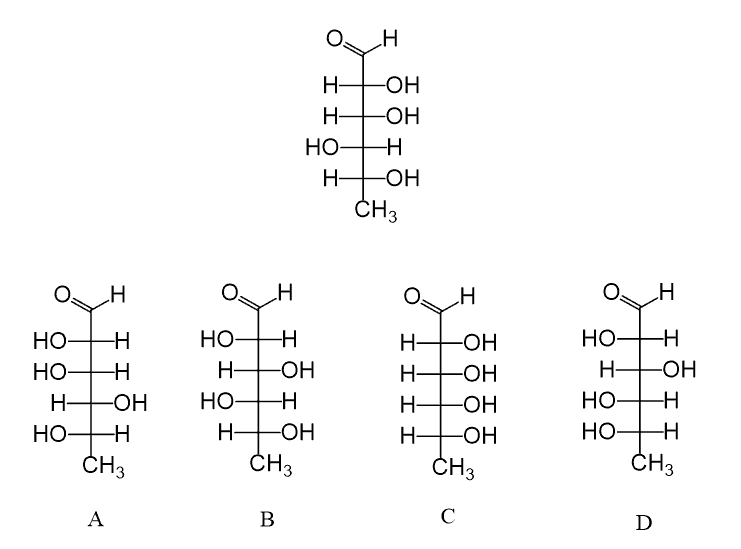
Fischer projections are especially useful in drawing carbohydrates since they contain multiple chiral centers, which are more time-consuming to draw.
For example, glucose, one of the most common and important carbohydrates, also used extensively for the initial studies, was found to exist naturally as one enantiomer designated as the D isomer. The enantiomer, L-glucose, can still be prepared synthetically:

What can be done with a Fischer Projection, and what is disallowed?
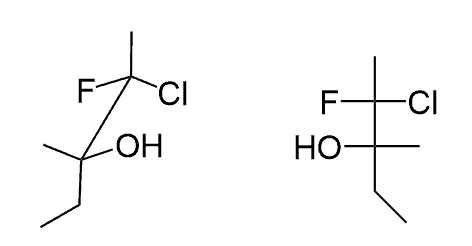
When redrawing a Fischer projection shown from a different direction, you are allowed to rotate the molecule by 180o , but NOT by 90o.
The reason for this is the fact that the absolute configuration of the chirality centers must be retained since it is the same molecule. This is only possible if the horizontal groups stay horizontal and the vertical groups stay vertical as well:

If you rotate the molecule by 90o, the horizontal groups get in the vertical positions, which in Fischer projections means that they are now pointing away from you. This changes the absolute configuration from S to R, and therefore, you are showing the enantiomer if the molecule:

This video is a fragment of a 108-question, multiple-choice Stereochemistry quiz and explains the rules for drawing Fischer projections in the context of determining the relationship between given compounds. The quiz comes with a 3-hour video solution.
Stereochemistry Practice Problems Quiz
In the following practice problems, you can practice converting Fischer projections to bond-line representation and assigning the R and S absolute configuration of chirality centers on Fischer projections.




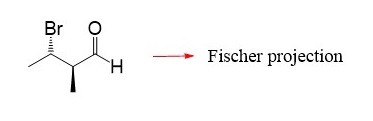


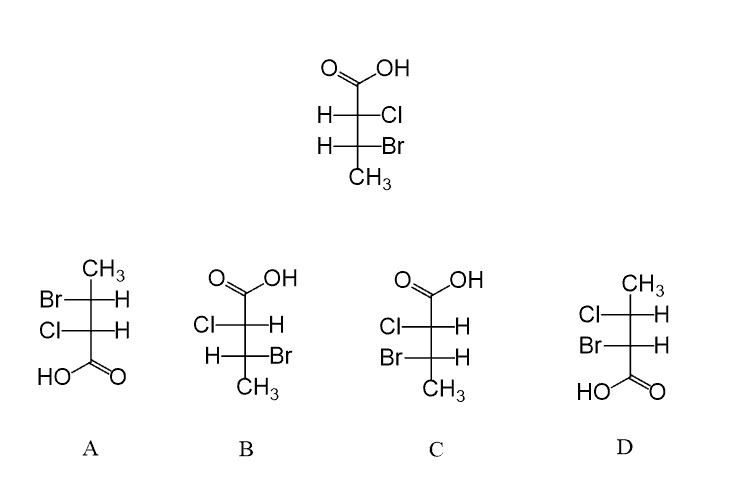
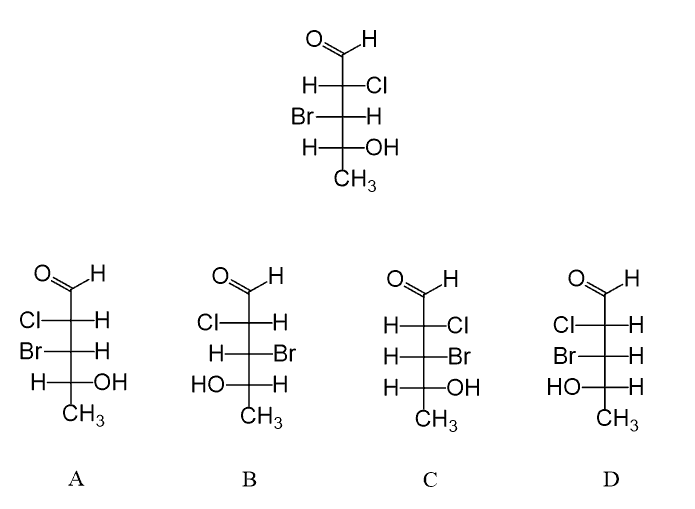
Good explanation. One question though, would it be accurate to say that the group pointing forward is always going to be on the right?
No, it always depends on the direction you are looking from. Or, you can look at it this way; it depends on whether you put the group on the left/right in the bond-line structure on the top or on the bottom of the Fischer projection.
Thank you for this 🙂
I like the right and left hand notation, so helpful.