You have learned and seen in general and organic experimental sections that compounds can be separated using techniques such as filtration, distillation, crystallization, etc. On the basis of all these techniques is the fact that the compounds have different physical properties. For example, distillation relies on the difference in the boiling points, whereas filtration and crystallization rely on different solubilities.
Now, when the two components of the mixture are enantiomers, separation becomes a lot more challenging because they have identical physical properties melting point, boiling point, and solubility. One of the features that distinguishes enantiomers is how they interact with plane and circularly polarized lights. Remember that enantiomers rotate the plane of polarized light to the same extent but in opposite directions. For example, the specific rotation of (R)-2-butanol and (S)-2-butanol are -13.52 o and +13.52o respectively.

This feature, however, can be used only for distinguishing rather than separating enantiomers. So, how do we separate enantiomers then?
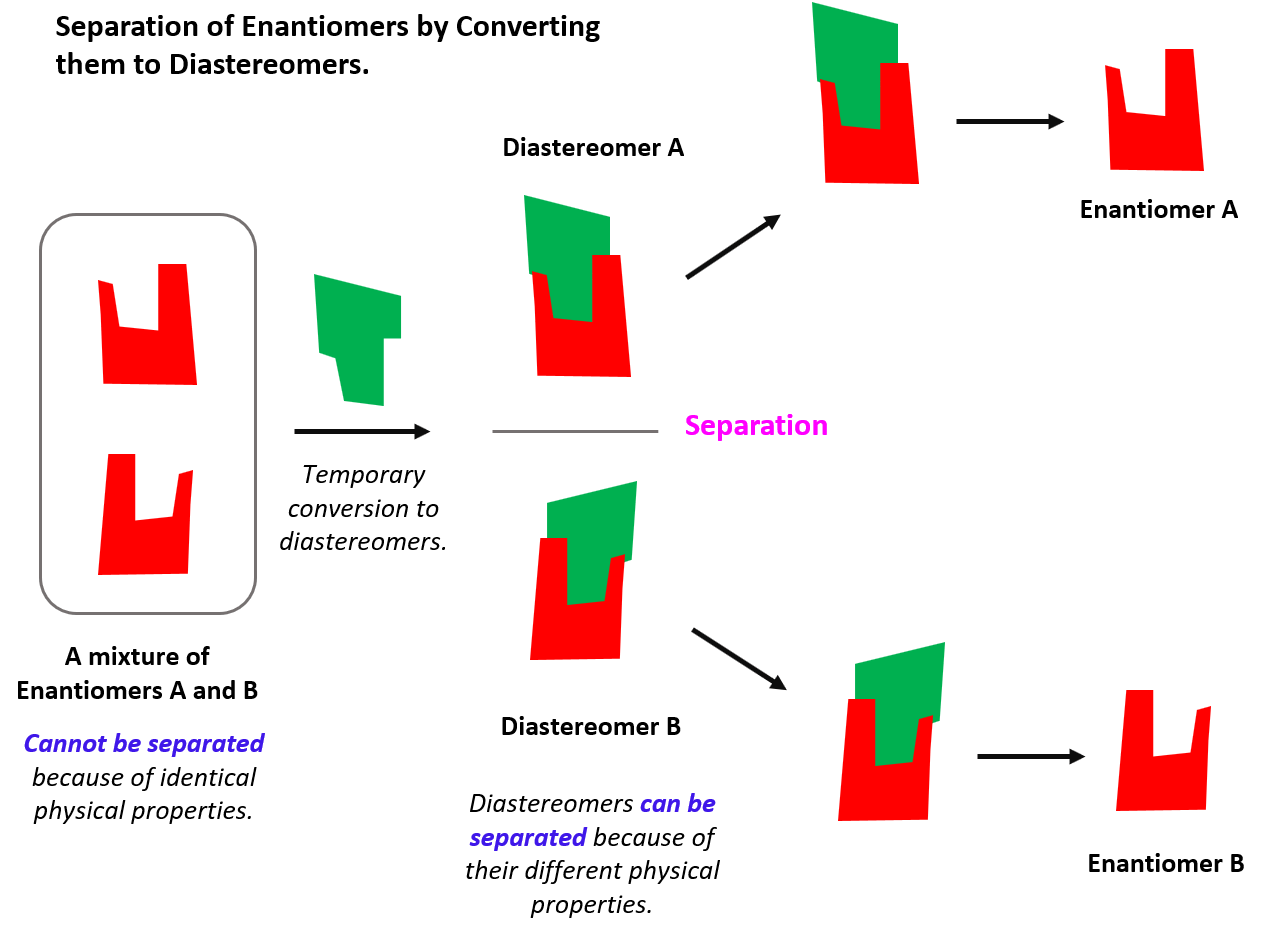
One of the most common ways, at least for educational properties, is their temporary conversion to diastereomers. Recall that diastereomers have different physical properties and can be readily distinguished and separated. Once converted to diastereomers, we separate them back to the original enantiomers:
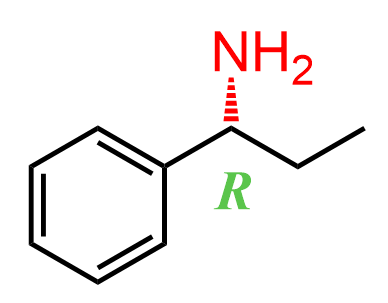
For example, let’s say we need to synthesize the R isomer of the following amine:
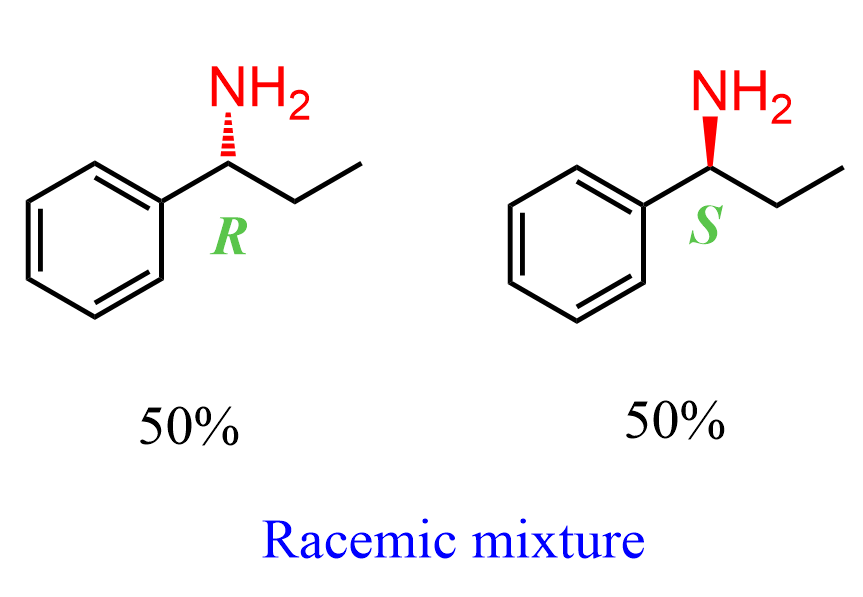
The problem is that very often, the synthesis of chiral compounds leads to a racemic mixture, which means the amine will be formed in a 50:50 mixture of the R and S enantiomers:
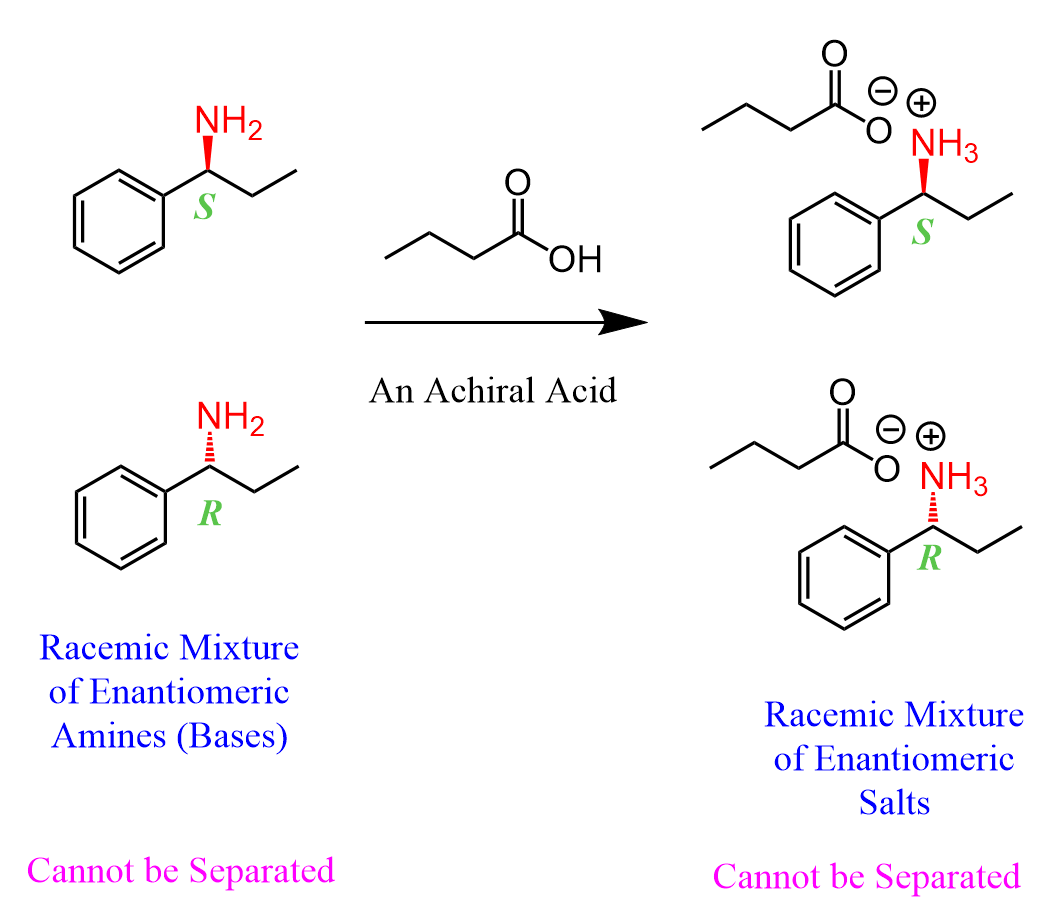
Amines are bases, and when reacted with acids, a salt is formed. One useful property of salts is that they can be hydrolyzed back to the component acid and the base. Now, if we react the amine with a regular acid, for example, butanoic acid, a racemic mixture of the salt will be formed, and we won’t be able to separate them:
Therefore, we need to use a chiral carboxylic acid so that the resulting salt has two or more chiral centers, where one of them is different (coming from the enantiomer amines) and the others are identical (coming from the acid). In other words, using a chiral acid allows for preparing a mixture of diastereomers, which can now be separated because of their different physical properties. So, instead of butanoic acid, we can use S or R-2-methylbutanoic acid:

This strategy could also be used if the initial mixture consisted of enantiomeric carboxylic acids. The only difference is that in this case, we’d use a chiral amine to temporarily prepare a mixture of diastereomeric salts.
Check Also
- How to Determine the R and S Configuration
- The R and S Configuration Practice Problems
- What is Nonsuperimposable in Organic Chemistry
- Chirality and Enantiomers
- Diastereomers-Introduction and Practice Problems
- Enantiomers vs Diastereomers
- Cis and Trans Isomers
- E and Z Alkene Configuration with Practice Problems
- Enantiomers vs Diastereomers
- Enantiomers, Diastereomers, the Same or Constitutional Isomers with Practice Problems
- Configurational Isomers
- Optical Activity
- Specific Rotation
- Racemic Mixtures
- Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
- R and S Configuration of Allenes
- Converting Bond-Line, Newman Projection, and Fischer Projections
- Stereochemistry Practice Problems
- Stereochemistry Practice Quiz
![]()

