A functional group is a specific atom or group of atoms within a molecule that largely determines its chemical reactivity, physical properties, and behavior in reactions. These groups are the key to understanding how and why organic molecules interact the way they do. Recognizing and mastering functional groups is one of the first and most essential skills for anyone studying organic chemistry.
In this post, we’ll break down the structures, naming patterns, and interesting real-life applications of the most important functional groups in organic chemistry. You’ll see how small changes in molecular structure, such as replacing a hydrogen atom with an -OH, -NH₂, or -COOH group, can drastically alter a compound’s properties and uses.
Let’s begin with the simplest class of organic compounds, the hydrocarbons, and build our way up from there.
Hydrocarbons
These, as the name suggests, are compounds that consist of carbon and hydrogen atoms. And depending on the connectivity and bonding types, we divide them into five main categories:
Alkanes, alkenes, alkynes, cycloalkanes, and aromatic compounds.
Alkanes
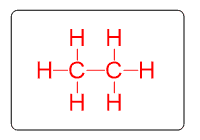
Alkanes are the first group of organic compounds. They are made of carbons and hydrogens that are only connected with single (σ) bonds.
You must know the first ten alkanes and their names, as they are on the basis of naming and understanding the structure of any other functional groups.

Key features: all the carbons are sp3-hybridized, only single bonds, and all the bonds are non-polar, therefore, alkanes and all the other hydrocarbons are non-polar, hydrophobic molecules.
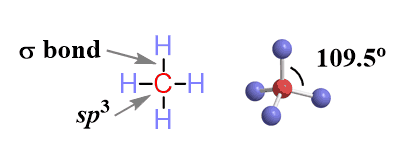
It will also be helpful to know the general formula of alkanes, which is CnH2n+2. For every n carbon, the molecule has 2n+2 hydrogens.
For example, propane has 3 carbons, therefore it has 2 x 3 + 2 = 8 hydrogens – C3H8.
Alkenes
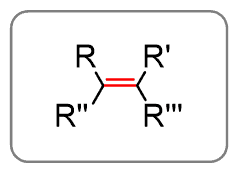
Alkenes are similar to alkanes with the only difference being the presence of a double bond.
The R groups can be H or hydrocarbon chains (see below about the alkyls)
This double bond changes the suffix from -ane to -ene.
For example:
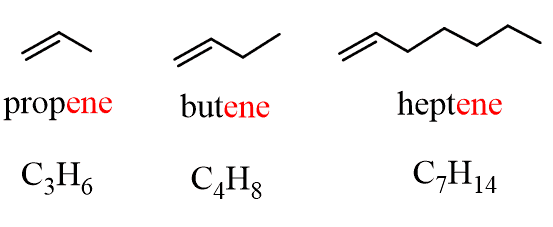
The two carbons with the double bond are sp2–hybridized, and the geometry is trigonal planar with a 120o angle between the atoms. The double bond is made of one σ and one π bond.
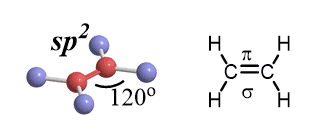
The presence of a double bond decreases the number of hydrogens per carbon atom and the general formula changes from CnH2n+2 to CnH2n.
Alkynes
The difference between alkynes and alkenes is the change of the double bond to a triple bond. The ending changes from -yne:

Alkynes have two hydrogens less than the corresponding alkenes and therefore the general formula is CnH2n-2
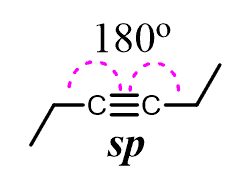
The hybridization of triple-bonded carbons is sp which corresponds to the liner geometry – 180o:
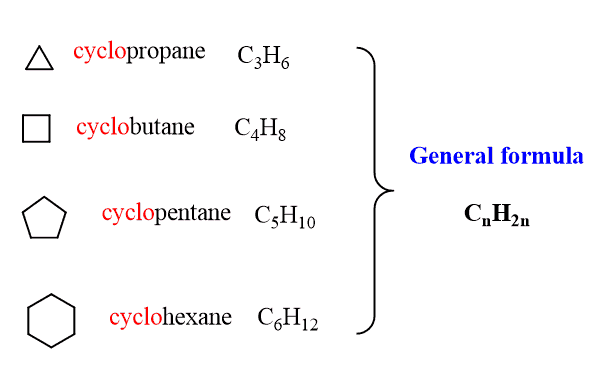
Cycloalkanes
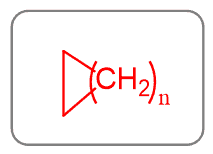
Cycloalkanes have the same hybridization as the alkanes and only single bonds are present in the molecule.
One important difference is the change of the general formula to CnH2n as there are no terminal CH3 groups:
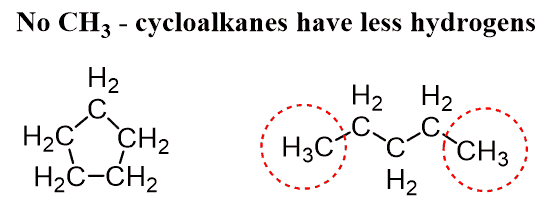
They are named just like the alkanes by adding the cyclo– prefix:
Aromatic compounds
This is a big class of compounds with specific physical and chemical properties, and there is a different chapter dedicated to studying all of that. However, when learning the functional groups, in most Organic 1 classes, you would only need to recognize the most common aromatic molecule, which is benzene:
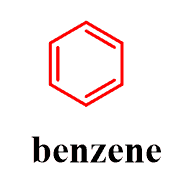
What are the key patterns to recognize here? First, remember that it is cyclic (all the aromatic compounds are cyclic), and it has a double bond on every second carbon. It goes interchangeably single-double. Here are some important molecules that contain aromatic rings:
Notice that not every cyclic compound with a double bond(s) is aromatic – it must have a certain number of alternating π bonds.
For example, a ring with one double bond is a cycloalkene:
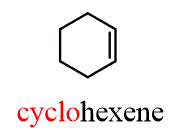
The definition of aromatic compounds is more complex, so for now, just remember the benzene ring.
Now, let’s switch to other functional groups that contain heteroatoms (any atom except carbon and hydrogen).
Alkyl Halides
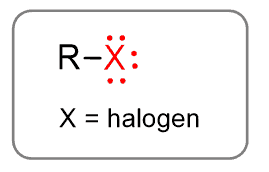
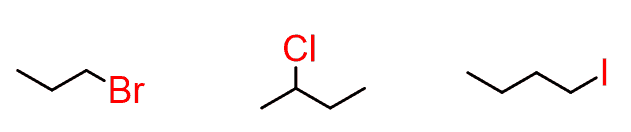
Let’s first understand the word alkyl. If we draw any alkane and remove one of the hydrogens, this brings the possibility of placing other atoms or groups in that position. For example:
The hydrocarbon chain is often represented with the letter R to keep the structure short. This R is what we call an alkyl group, which by itself is shown with a line on the position where we removed the hydrogen:

So, alkyl halides have a general formula of R-X, where X can be any halogen atom.
Nitriles
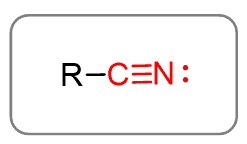
Alkyl groups together with the -CN (cyano) group make the nitriles:
Acetonitrile is a common solvent in organic laboratories, and many other nitriles are used in synthetic polymers and glues.
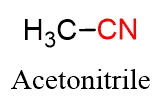
Azides
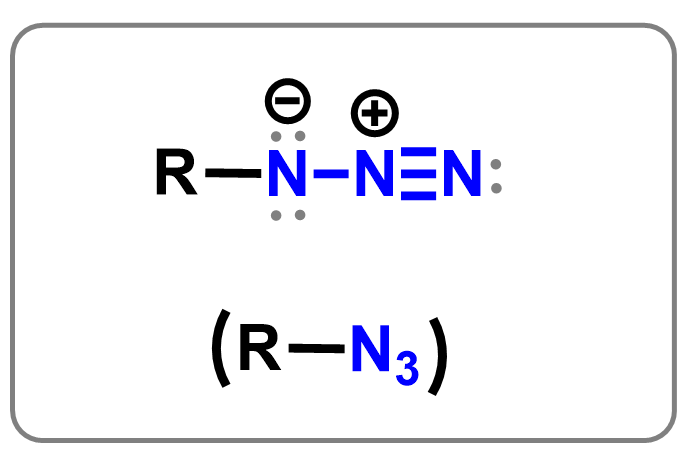
Azides are functional groups with the formula –N₃, consisting of three nitrogen atoms in a linear arrangement. They are generally used as intermediates in organic synthesis for preparing amines and other nitrogen-containing compounds. Azides are especially important in click chemistry, where they react with alkynes in a copper-catalyzed cycloaddition to form triazoles, which are types of aromatic compounds. Due to their explosive potential, some azides must be handled with care in the lab.
Nitro Compounds
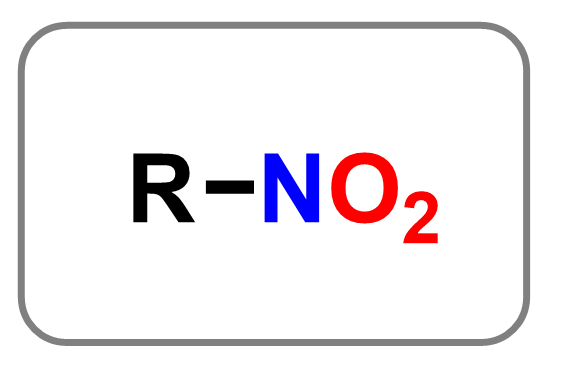
Nitro compounds contain the –NO₂ functional group, where a nitrogen atom is bonded to two oxygen atoms. One of the oxygens is connected via a double bond, and the other via a single bond (with a negative formal charge). Nitro groups are commonly found in explosives (like TNT), dyes, and pharmaceutical intermediates. You will probably not see them again in organic chemistry one, but they do come around in organic chemistry as this group is strongly electron-withdrawing, which makes nitro compounds highly reactive in electrophilic aromatic substitution and reduction reactions.
Alcohols
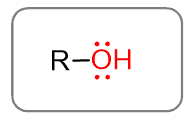
If instead of the halogen, we put an OH (hydroxyl group) on an alkyl halide, we get an alcohol.
For the general formula, we have R-OH, and below are some common examples:
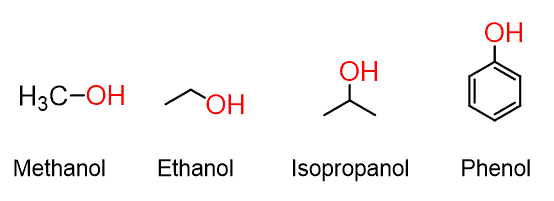
Methanol – an important organic solvent, very toxic, causes blindness
Ethanol – the alcohol in beverages
Isopropanol – this is the rubbing alcohol
Phenol – an important aromatic alcohol with many applications
Peroxides
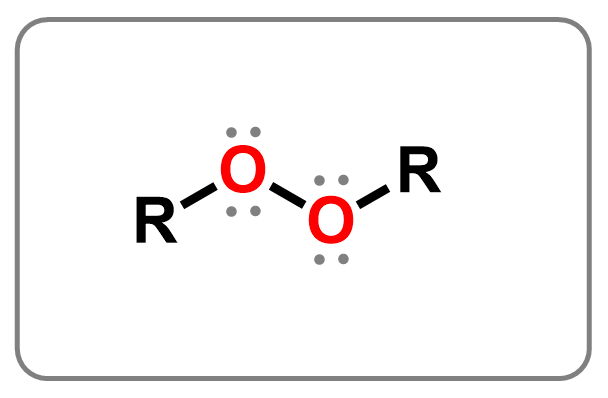
Peroxides are functional groups with an –O–O– single bond, where two oxygen atoms are directly connected. This bond is weak and highly reactive, which makes peroxides useful as oxidizing agents and radical initiators in organic reactions, especially in polymerization. A well-known example is hydrogen peroxide (H₂O₂), a simple inorganic peroxide commonly used as a disinfectant, bleaching agent, and antiseptic in everyday life.
Have you noticed that hydrogen peroxide is usually sold in dark brown bottles? That’s because it decomposes in the presence of light, releasing oxygen gas. The dark bottles help protect it from light and slow down this breakdown process.
Epoxides
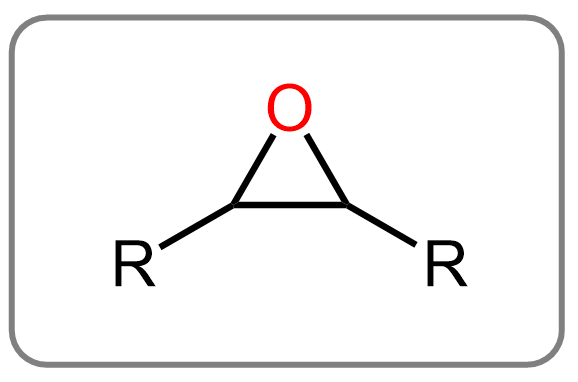
Epoxides are three-membered cyclic ethers with an oxygen atom bonded to two adjacent carbon atoms.
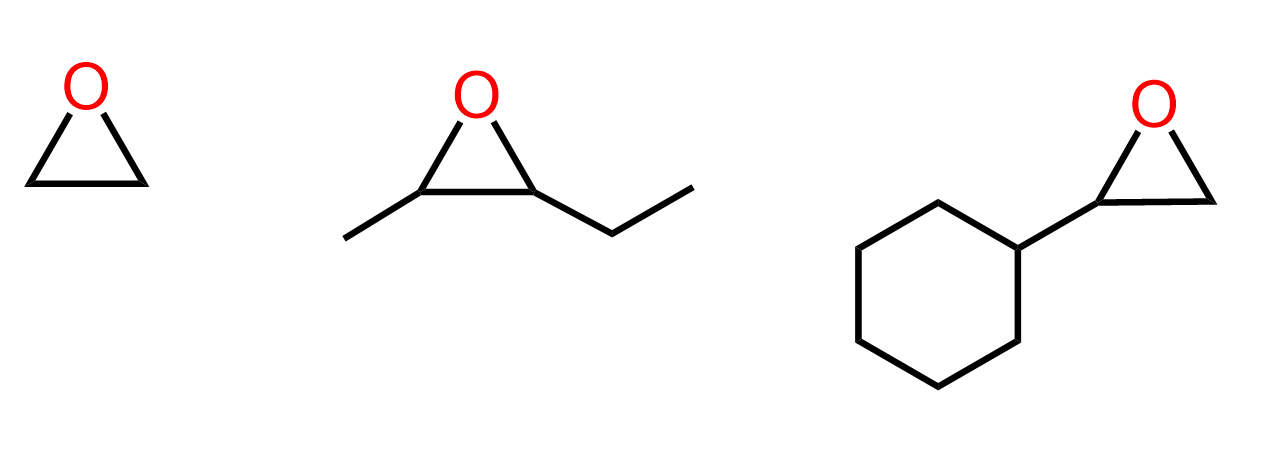
Because of the ring strain in the three-membered structure, epoxides are highly reactive compared to regular ethers. They readily undergo ring-opening reactions with nucleophiles, making them useful intermediates in organic synthesis and functional group transformations.
Thiols
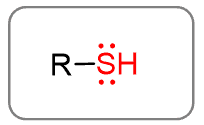
The sulfur analog of alcohols is the thiol functional group:
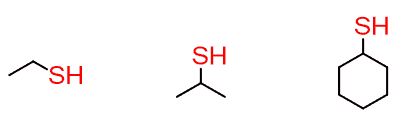
Thiols are reducing agents and play an important role in preventing oxidative damage in cells. One noticeable example is glutathione, which is an antioxidant in plants and is used by humans as a supplement.
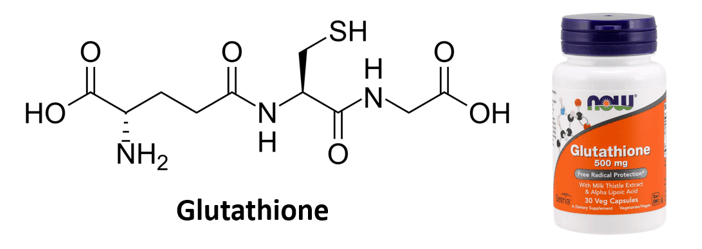
What other functional groups do you recognize in glutathione?
The SH (mercapto group) group is also present in the amino acid cysteine:

One bad thing about the thiols is their smell, especially when it is a small molecule like ethane thiol. What was the worst restroom you went to?
Ethers
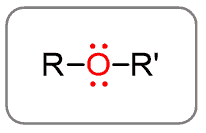
Ethers are different from alcohols in that the hydrogen of the hydroxyl group is replaced with another alkyl group. So, to recognize the ether in an organic molecule, look for the bridging oxygen:

The most commonly used ether is the diethyl ether which is an organic solvent and was previously used as a general anesthetic.

Thioether (Sulfides)
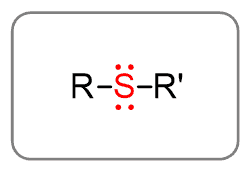
Since sulfur is often divalent (makes two bonds) it also serves as a bridge for two alkyl groups:
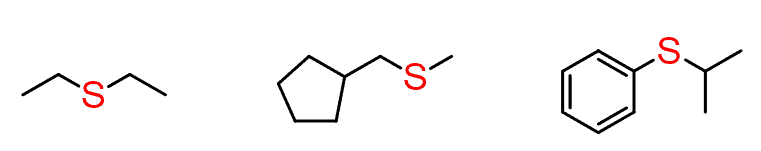
These are called thioethers or sulfides.
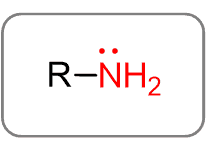
Amines
Amines are the derivatives of ammonia (remember NH3 from General Chemistry). Replacing one hydrogen of ammonia with an alkyl group forms an amine with a general formula of R-NH2:

Depending on how many alkyl groups are connected to the nitrogen, we have primary, secondary, and tertiary amines:

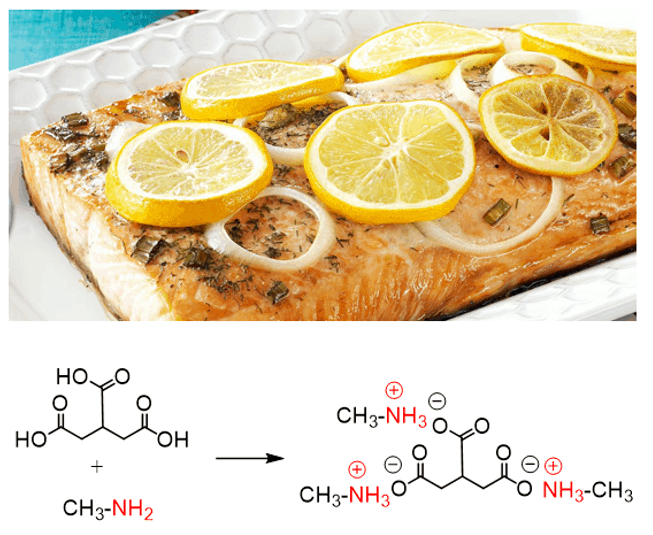
Amines have the characteristic fish smell. They are considered organic bases since the nitrogen can be protonated even with relatively weak acids. When cooking fish, lemon is often added to neutralize the smell because it contains citric acid which reacts with the amines in the fish:
Carbonyl-containing functional groups
Carbonyl (C=O) is an extremely important and common group that is part of many functional groups:

Aldehydes
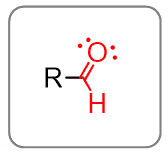
Connecting a hydrogen with a carbonyl group gives an aldehyde:
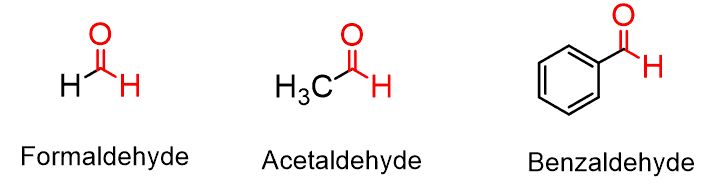
The simplest aldehyde with one carbon atom is formaldehyde, followed by acetaldehyde.
Formaldehyde is widely used in medicine as a preservative agent and also as a precursor for the synthesis of many chemicals.
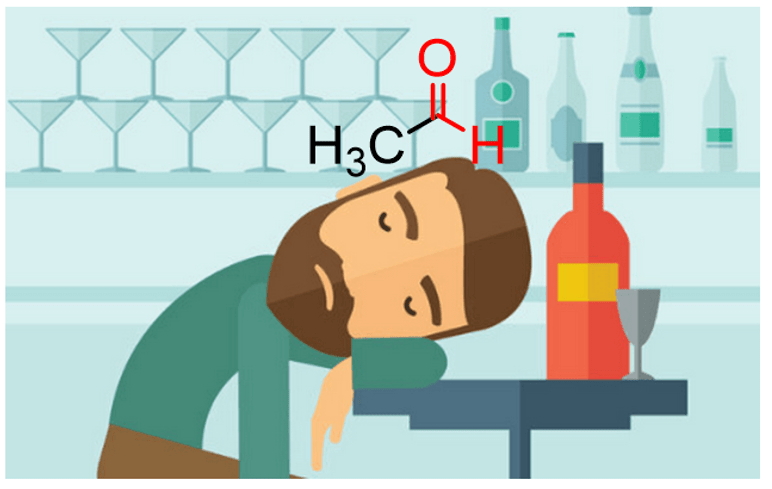
Acetaldehyde is a naturally occurring aldehyde that is also produced on a very large scale for the chemical industry. It is also produced in the human body from ethanol by the Alcohol dehydrogenase enzymes and is partly responsible for the cause of the hangover.
Ketones
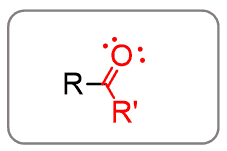
Putting alkyl groups on both sides of the carbonyl switches from aldehydes to ketones:
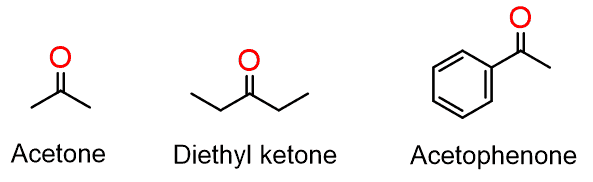
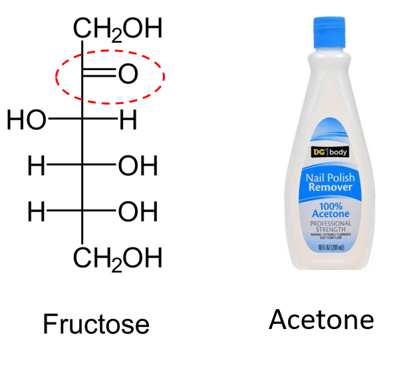
Some of the ketones, such as ketoses (e.g., fructose), are of great biological importance. Acetone is a commonly used solvent in organic labs and a nail polish remover.
Carboxylic acids
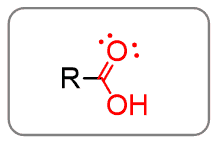
The combination of the carbonyl with OH gives the carboxylic acid functional group:
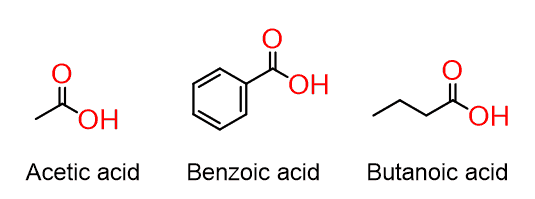
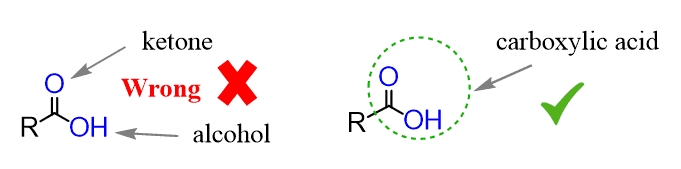
Remember, however, that you cannot indicate them as two different functional groups! If they are together, it is the carboxy group.
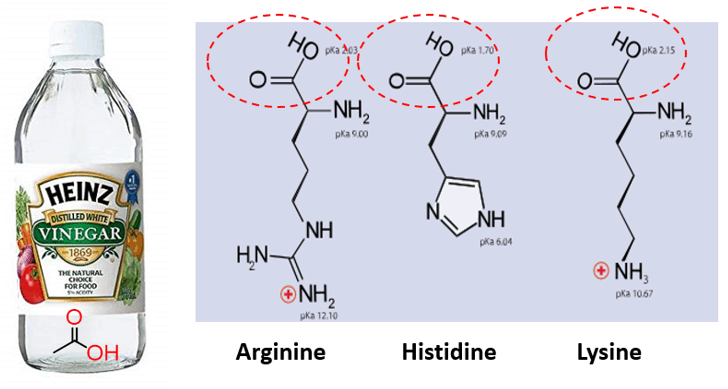
As the name suggests, these are organic acids and therefore, are very common and widely used for different applications. For example, vinegar is a ~5% aqueous solution of acetic acid. Carboxylic acids are also part of the amino acids, which are central in life:
Esters
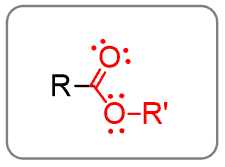
Having another alkyl group in place of the hydrogen of carboxylic acid makes the esters:
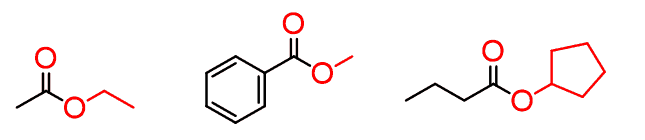
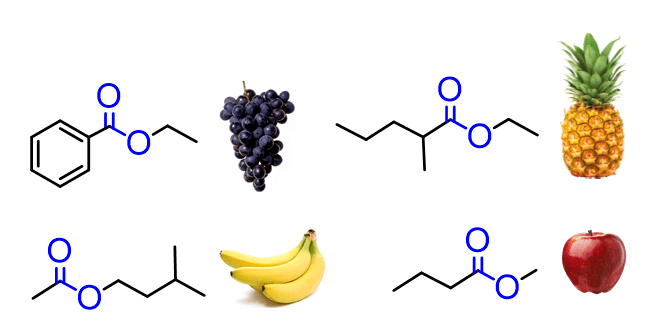
Esters are extremely abundant, occurring both naturally and synthetically. Most esters have a characteristic smell responsible for the aroma of fruits, flowers, wine, perfumes, etc.
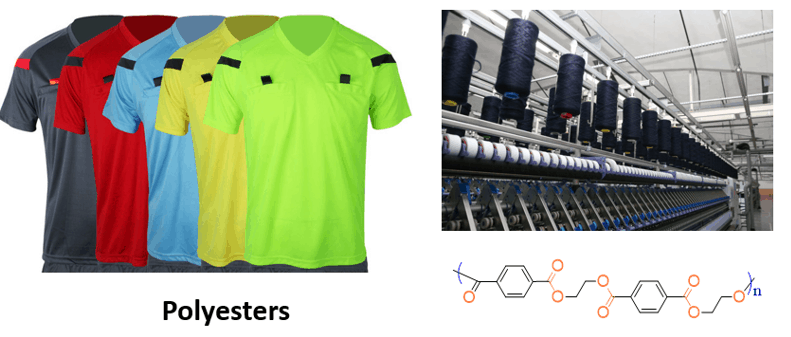
A large portion of industrial applications goes to the synthetic polyesters. Polyesters such as polyethylene terephthalate (PET) are widely used in clothing, plastics, furniture, tires, and many other products.
Amides
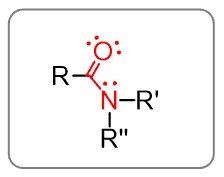
Amides are the combination of the carbonyl and amines:
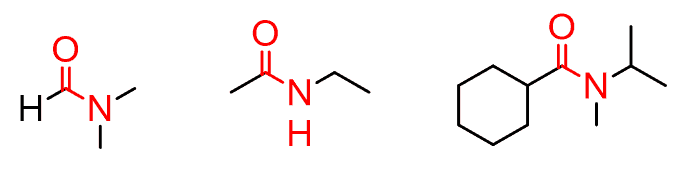
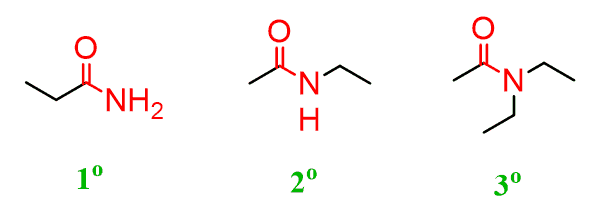
Just like for the amines, depending on the number of carbons connected to the nitrogen, we have primary, secondary, and tertiary amides. Notice that the carbonyl carbon is also counted:
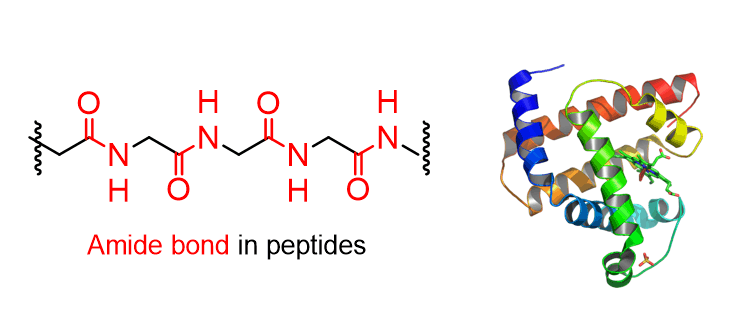
Amides are essential in chemistry and biology as they are part of many peptides and nucleobases. In fact, the amide is also known as the peptide bond since it is the linkage of amino acids in peptides and proteins.
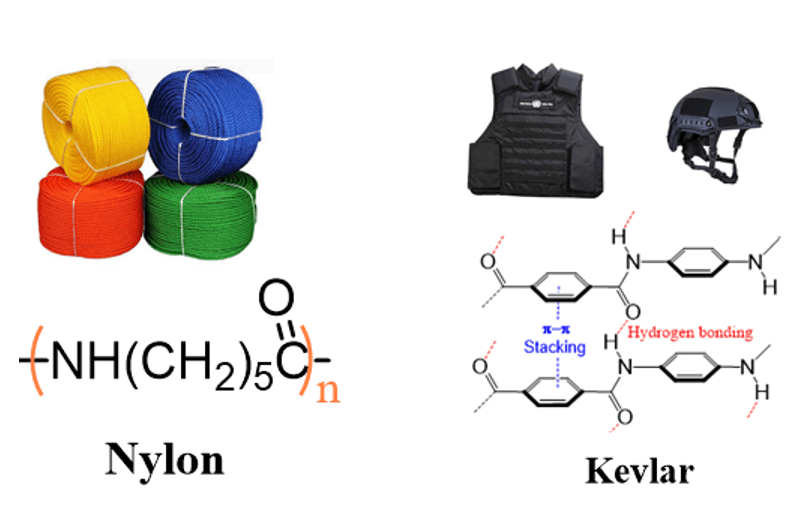
As expected, amides are very stable and that is why they are also used in synthetic polymers such as nylon and Kevlar, used in the production of bullet-proof material:
Acid Chlorides
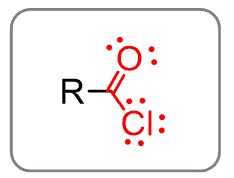
Acid chlorides are derivatives of carboxylic acids where the acidic proton is replaced by chlorine:
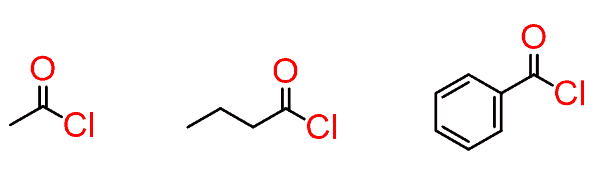
These are very reactive and are mainly used in organic synthesis.
Functional groups with two carbonyls
You may not see them as often, but acid anhydrides and imides are also important functional groups in organic chemistry:
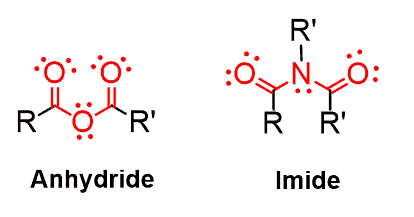
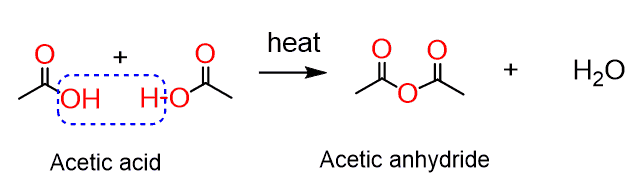
Anhydrides are prepared by removing one water molecule (dehydrating) from two carboxylic acids, which is indicated by the name of the functional group:
Finally, all the common functional groups are summarized in the following table:

Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.






Thumbs up! Nice.
Thank you.
We appreciate it, thanks
Glad it is helpful.