Before we start talking about naming bicyclic compounds, let’s first go over some terminology for their classification.
Bicyclic compounds are a specific type of polycyclic compounds, classified as compounds that contain more than one ring with at least two common atoms. The common atoms connecting the rings in a bicyclic compound are called bridgehead carbons:

Bicyclic compounds are in turn divided into fused– and bridged bicyclic compounds. It is fused when the bridgehead carbon atoms are adjacent and bridged when they not.

So, in bridged bicyclic compounds, the joint carbons are farther apart and when they get next to each other (connected) we have fused bicyclo compounds. The extreme end of this relationship is when there is only one common carbon, and these are called spirocyclic compound:
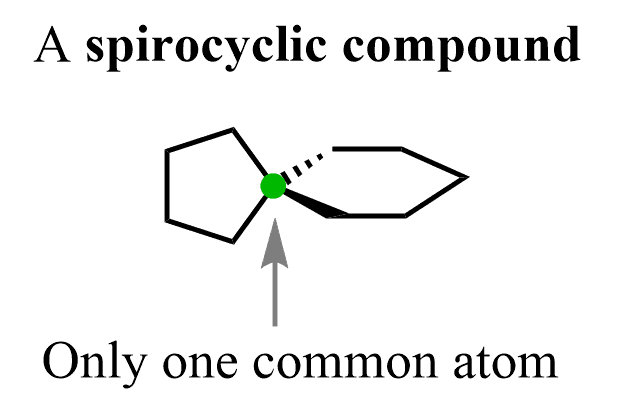
However, our focus is going to be on the bicyclic compounds and their nomenclature.
Naming Bicyclic Compounds
The first rule for naming bicyclic compounds is to start numbering from one of the bridgehead carbon atoms. Next, you need to decide the correct direction. For example, what is the correct direction for numbering in the following compound?
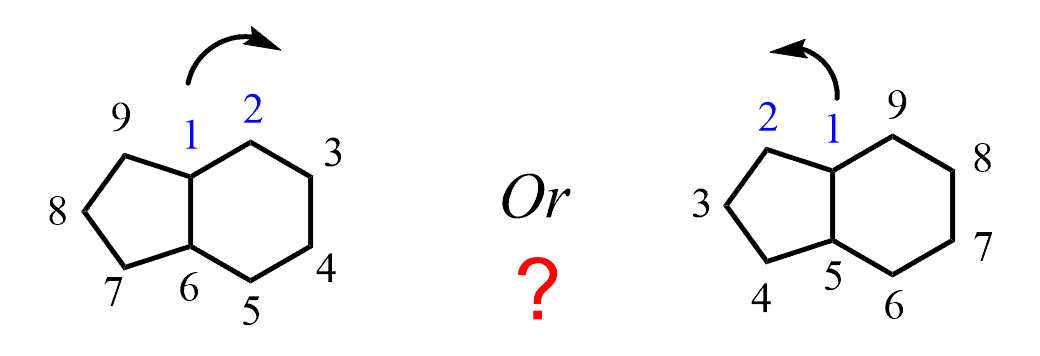
For this, you need to remember that the rings are numbered according to their size – the one with more carbon atoms is numbered first, then the second and etc. So here, we will go clockwise numbering the ring on the right and the one on the left:

You may wonder why I call them “a ring on the right” and “a ring on the left” instead of cyclohexane and a cyclopentane, and the reason is that they are not actually two rings of cyclohexane and cyclopentane but rather a whole structure with 9 carbon atoms. In fact, the parent chain of bicyclic compounds is given based on the total number of carbons in all the rings. So, this compound is named as nonan,e and the number of carbon atoms in each ring is designated as 4 and 3 rather than 6 and 5.
With this said, let’s put together the basic unit for naming bicyclic compounds. It is the word “bicyclo” followed by a square bracket with the number of carbon atoms in each ring:
Bicyclo[#C, #C, #C]parent chain
In the example above, we’d have bicyclo[4.3.0]nonane:

Notice again that the numbers in brackets are showing the number of carbon atoms in each ring excluding the bridgehead carbons.
One question you may wonder is the zero in the brackets. This is to specify that there are only two rings and not three (zero carbons in the third ring). For example, we could have another bicyclic nonane with three joint rings:
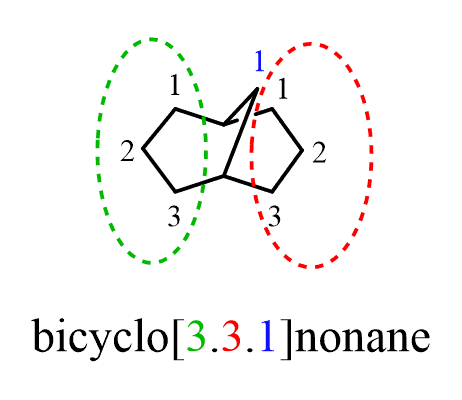
In this case, we have [3.3.1]nonane since there is a third ring with one carbon atom, while [3.3.0]nonane indicated only two rings.
Bicyclic Compounds with Substituents
The presence of substituents in a bicyclic compound doesn’t affect the main rule for numbering which is to start it from one of the bridgehead carbon atoms and move in the direction of the larger ring towards the other bridgehead carbon atom.
For example, despite the presence of a Br group in the left ring, the larger ring on the right gets the priority and is numbered first. The substituent is placed in the beginning of the name just like in the nomenclature of alkanes:

It is worth mentioning that there a chiral center, but the R and S notation for bicyclic compounds is mostly left out at least in the undergraduate curriculum.
Ring Priority in Bicyclic Compounds
During my classes, I have noticed an extremely effective way of making this topic a lot easier. What we did was giving a priority to the rings and coloring them accordingly.
For example, let’s consider this compound with quite a lot of carbon atoms.
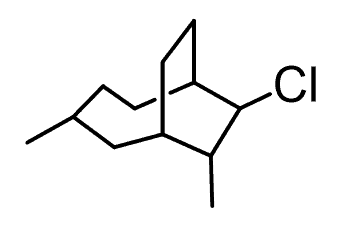
The first step to name it is to find the largest ring (ring priority 1). And this would be the one on the left since there are four carbon atoms vs two that the other have.
We will color code the ring priorities in the following way:
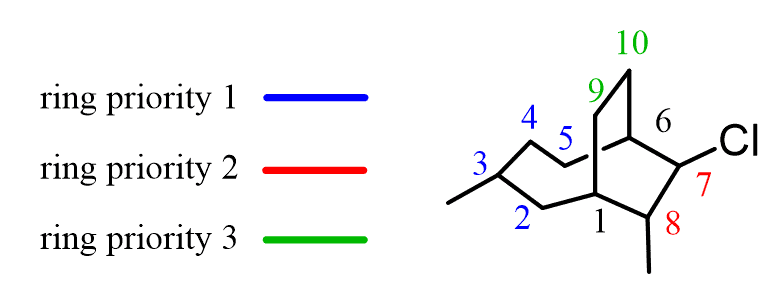
The blue ring gets priority one because of the greatest number of carbon atoms. The red ring is priority two because of the two substituents it has. Despite having the same number of carbon atoms, the green ring is only the third because of the lack of substituents.
Let’s put this together as a summary for determining the ring priority on bicyclic compounds.
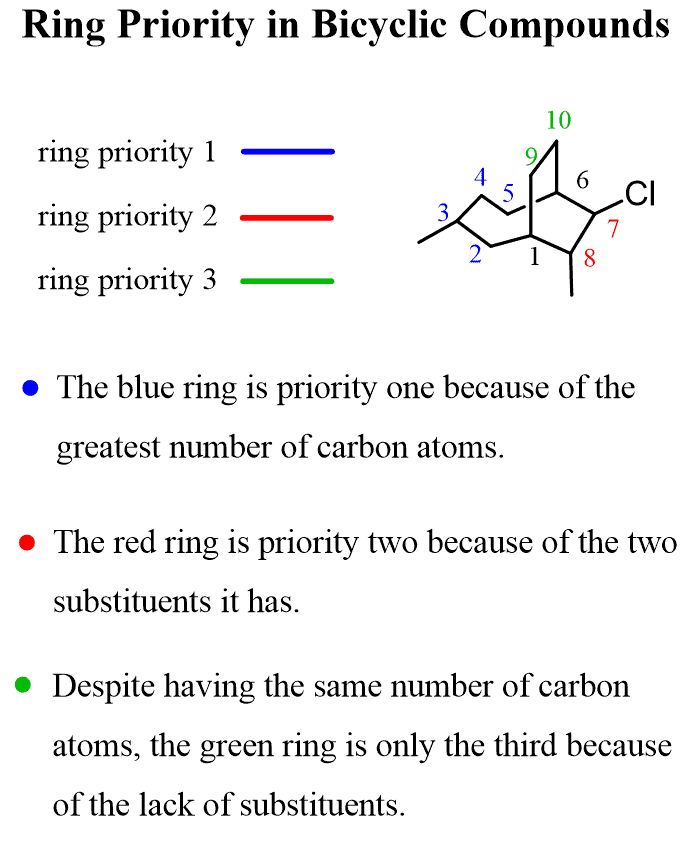
Now what if the rings are the same – they have the same number of carbon atoms? In this case, the one with a substituent gets the priority. For example:
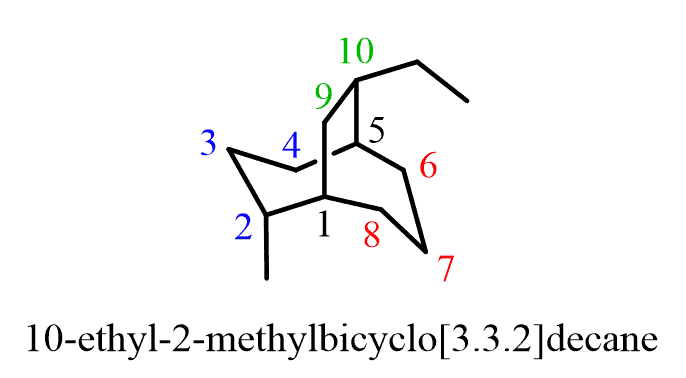
Both rings (left and right) have the same number of carbons. Therefore, the priority is given based on the methyl substituent. The ring on the top has only two carbon atoms and thus it gets the lowest priority despite the presence of a substituent which ring 2 lacks.
The last part of this post will be a summary chart for naming bicyclic compounds which you can also use to practice some additional examples below:



Good website
In the example of [3.3.1]nonane you write: “In this case, we have [3.3.1]nonane since there is a third ring with one carbon atom, while [3.3.0]nonane indicated only two rings.”
To me it appears that what you call rings should instead be called bridges.
Three bridges connect the bridgehead carbons, one 3 carbons long, another the same, and a third bridge 1 carbon long.
That in bicyclo[3.3.1]nonane there are 3 rings is questionable. Besides two five-carbon rings one may see a third eigth-carbon ring (carbons 1,2,3,4,5,6,7,8) but all those carbons were already counted in the previous rings.
Thanks
I think it is very good for everyone !!!
Very nicely explain all about nomenclature of bicyclo compounds with the help of most appropriate examples. Awesome 👌 👏 👍
How to decide whether we pick bigger ring or a smaller ring with unsaturation ?