Newman Projections of Butane
Let’s start today’s discussion with the definition of a conformation. The different spatial arrangements of the atoms that result from rotation about a single bond are called conformers or conformational isomers.
To illustrate the conformations of butane, we need to know the key principles of Newman projections. Recall that a Newman projection is a representation of the molecule looking through one of the C-C single bonds.

Because of the number of bonds and angles we can look at the molecule, we can have different Newman projections for the same molecule. Therefore, for every Newman projection, you need to specify the bond and the direction you are looking at. For butane, it is usually the C2-C3 central bond, and allows to study different conformations and energies of the molecule. So, let’s first draw a bond-line structure of butane showing the hydrogens on the middle carbons with a wedge and dash representation:
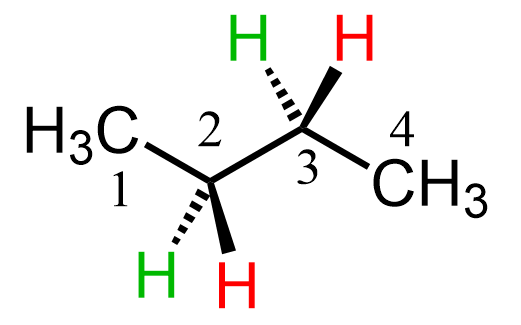
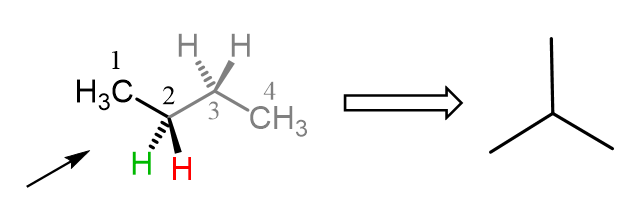
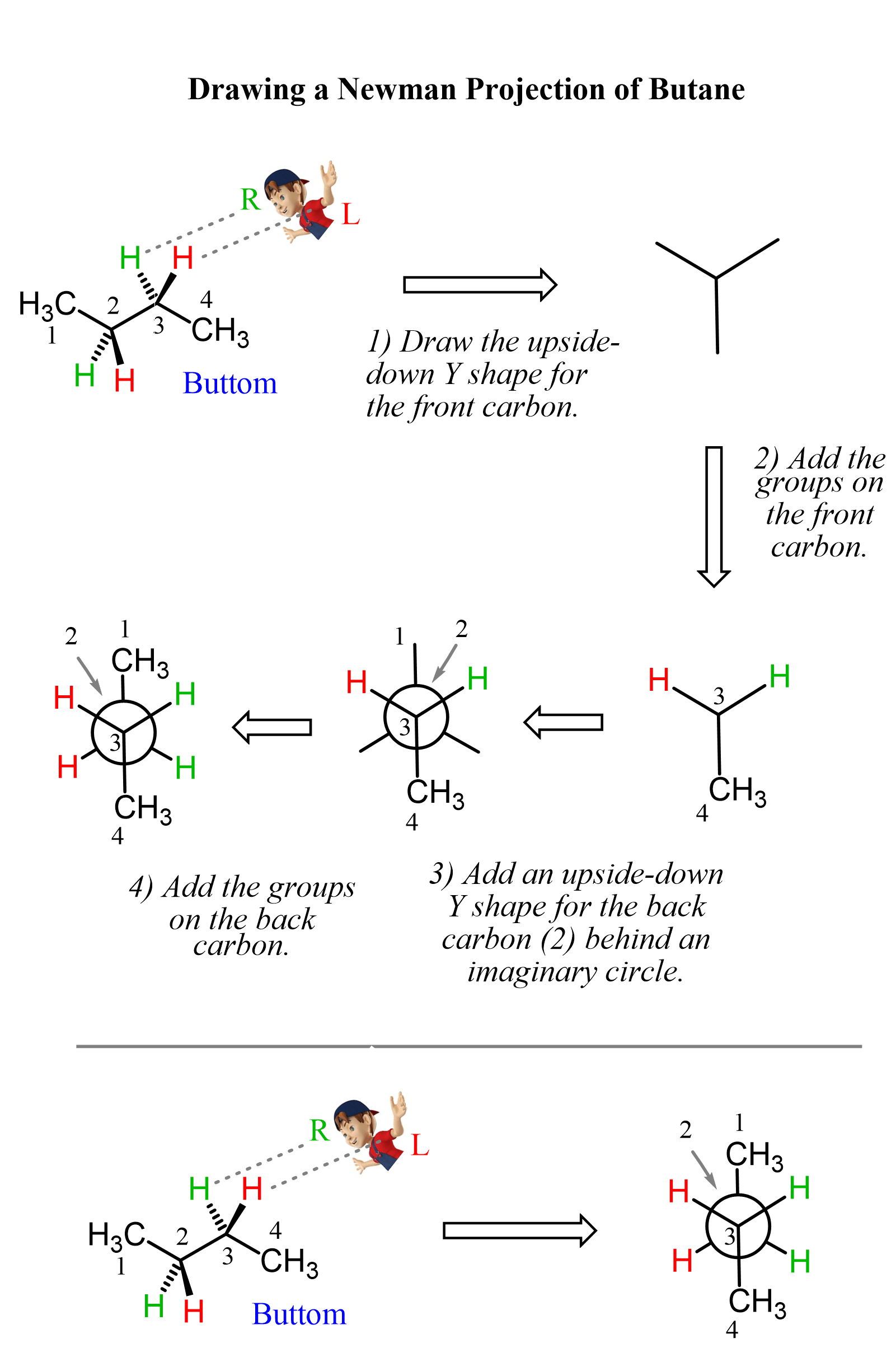
To draw a Newman projection of butane, we first need to choose the view angle. For example, we can do it based on the bottom-left corner, looking through the C2-C3 bond.
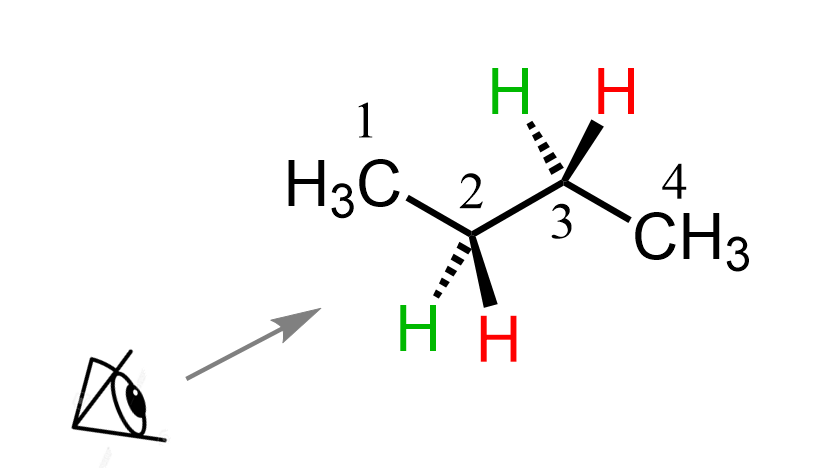
We will replace the simple eye symbol with this fellow to see which groups are pointing where i.e. up, down, top-left, top-right, etc.
What we can see here is that on the front carbon (carbon 2), we have the CH3 on the top, the wedge red hydrogen is on the bottom right, and the dash green hydrogen is on the bottom left.
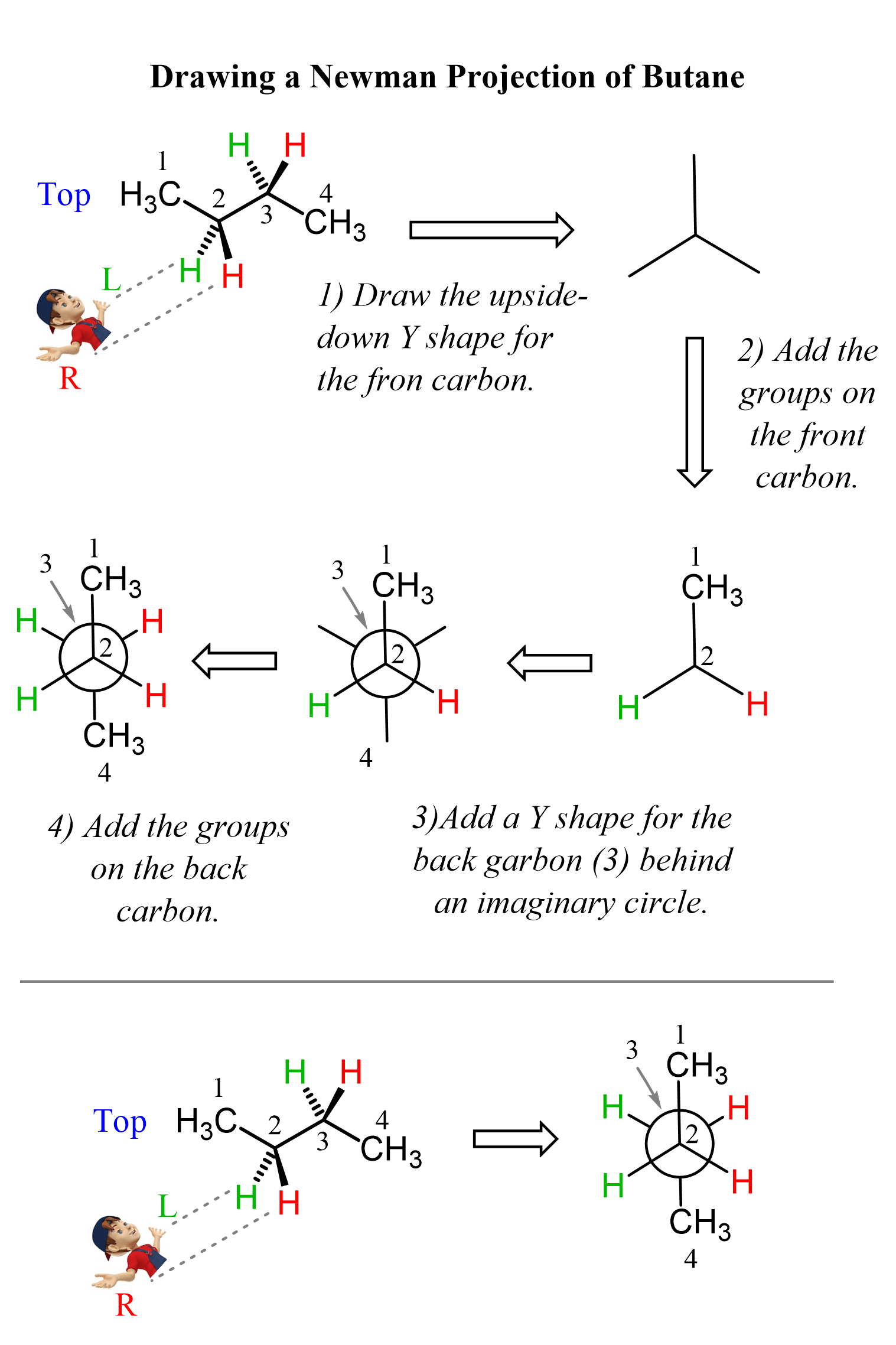
The shape of the front carbon from this direction is an upside-down Y or the Mercedes sign, and this is going to be the first part of drawing the Newman projection:
In the next step, we add the groups on the front carbon, then add a Y shape for the back carbon (carbon 3) behind an imaginary circle, and finally, add the groups on the back carbon. These steps are summarized in the following image:
Here is also a short video clip for better visualization when drawing the Newman projection of butane:
You may wonder why we chose this zig-zag pattern and not the one where the first methyl group is pointing down, or we are looking from the bottom-left instead of the bottom-right corner:
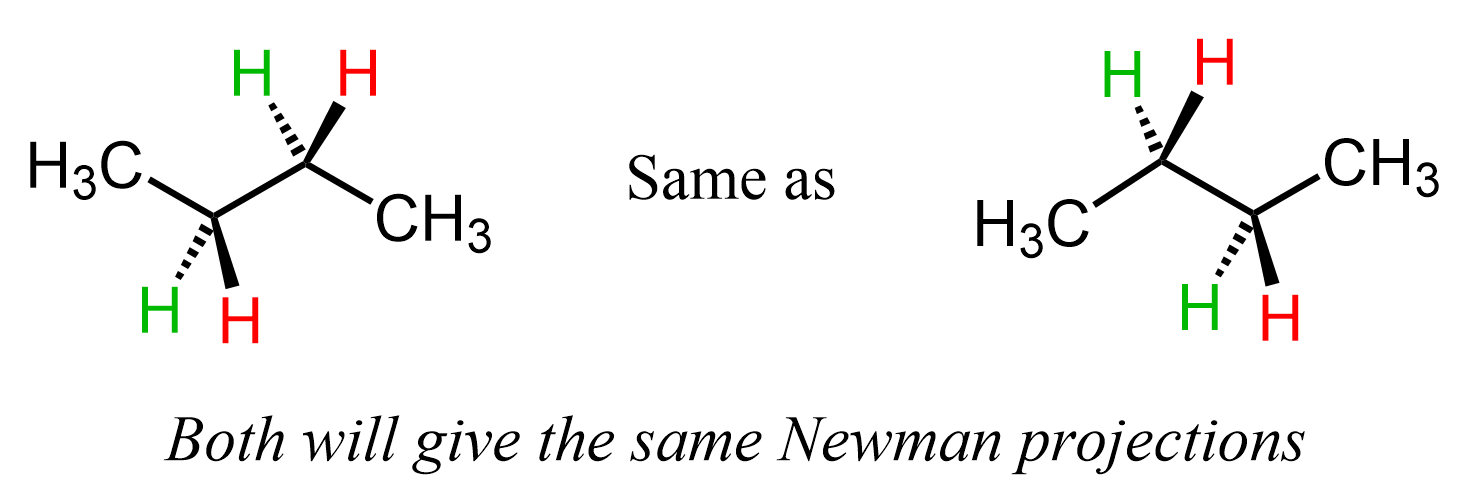
Do not worry about it – none of them is incorrect or has any advantage from just the drawing prospective. Remember that it is the same molecule, and can have multiple projections just like any other object. We just need to start from somewhere, and conceptually, all the projections and rotations about the given single bond will yield the same data.
Just to master the subject, let’s also draw the Newman projection looking from the top-right angle:
And a short video for this Newman projection as well:
It is important to mention that butane and most other molecules where there are no restricted factors such as, for example, a double bond or large groups, exist in infinite number of conformations. This is because the rotation about all the single bonds is continues and happens thousands or millions of times in a second depending on the temperature, media, and structure of the molecule.
We only focus on the distinct/extreme conformations with the largest energy gaps because these are where the molecule spends the most and least amount of time, and thus they define its physical and chemical properties. These are the staggered and eclipsed conformations, which we will discuss in the following section.
Before we get to the next part of this discussion, here is a short video summarizing the steps for drawing Butane’s Newman projections.
View this post on Instagram
General Stability of Eclipsed and Staggered Conformations
Like for any other tetrahedral carbon, the rotation about the single bond gives two distinct sets of conformation: staggered and eclipsed. Remember, in eclipsed conformations, all the atoms/groups on the neighboring carbons are aligned, whereas for the staggered conformations, the atoms/groups are at 60o:
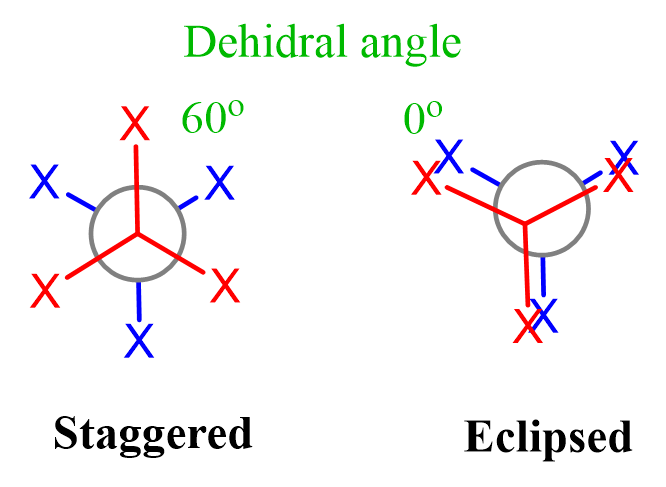
This angle between the atoms/groups on the neighboring carbons is called dihedral angle. So, for eclipsed conformations, the dihedral angle is 0o, and for the staggered conformations, it is 60o.
As we know from the conformational analysis of ethane and propane, staggered conformations are more stable than the eclipsed conformations because of steric and torsional interactions.
Although quite similar and often used interchangeably, the steric torsional interactions have different definitions. Sterics or steric interactions are the obstructions caused by the size of neighboring groups and atoms. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms.
Both steric and torsional interactions are observed mainly in eclipsed conformations because that is when the groups come into close proximity:

Very often and for butane in particular, the X’s represent either a hydrogen atom or a methyl (CH3) group. Because of the small size of hydrogen, the H:H and H:CH3 interactions are considered to be mainly torsional, whereas two methyl groups get in each other’s way and the steric factor is more significant. We will discuss these interactions in more detail below using the following table that summarizes the torsional and steric energies of eclipsing H and CH3 groups as well as those of gauche interactions which we will also discuss in this article:

Conformational Analysis of Butane
Conformational analysis is the study of the relative stabilities of different confirmations of a molecule. The conformational analysis of butane is based on the different conformations achieved via the rotation of the C2-C3 bond.
So, let’s arrange the conformations of butane on an energy diagram based on the energy of each type of combination between hydrogen and a methyl group.
On the left side, we will place the most stable (lowest energy) conformation where the two methyl groups are on the opposite side of the bond. This is called the anti conformation because the two largest groups are anti-periplanar. The anti conformation is one of the staggered conformations which are all going to be placed on the bottom section of the energy graph:
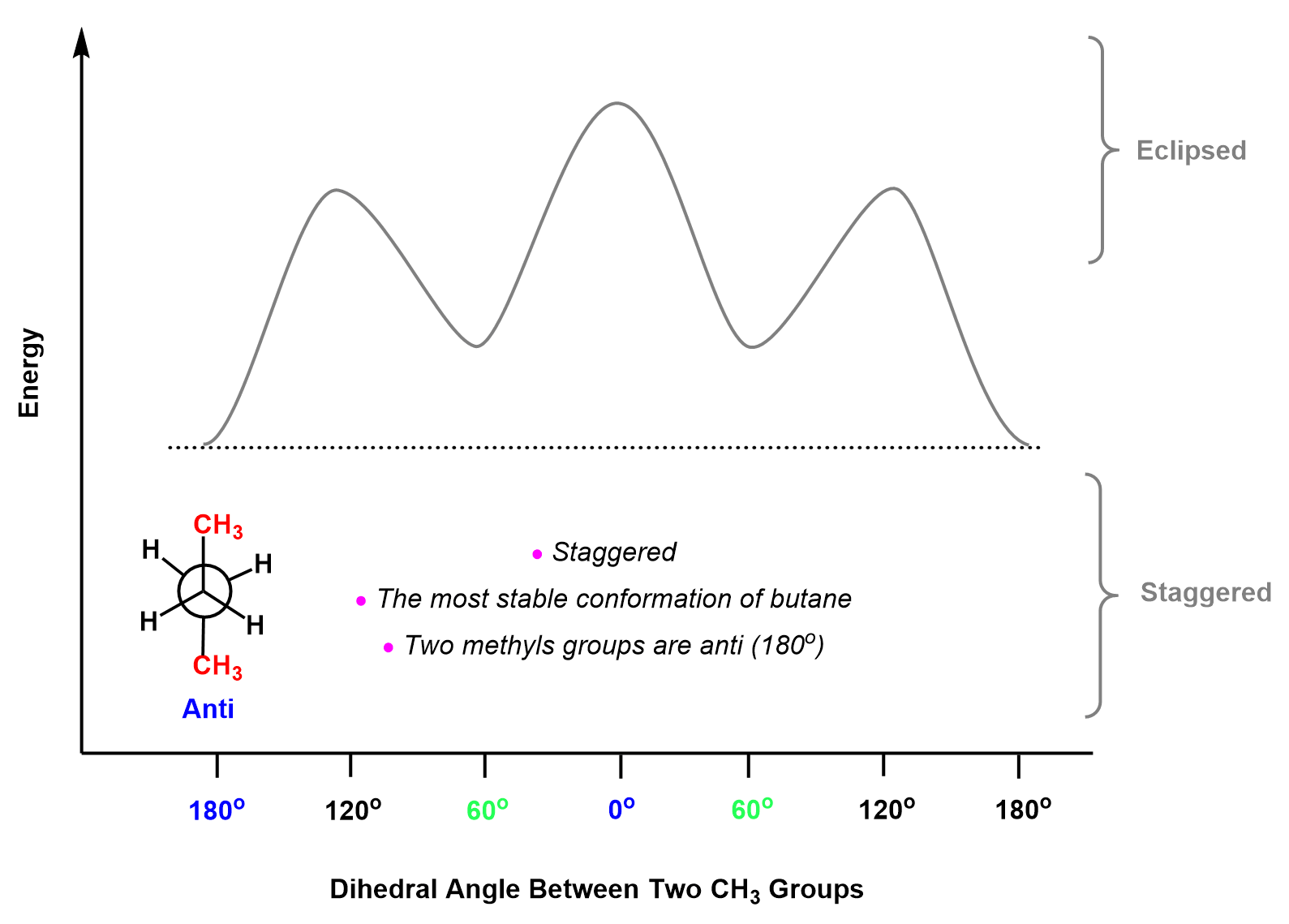
The next conformation is eclipsed and is achieved if we rotate one of the carbons by 60o. It does not matter which one, you do, so let’s rotate the back carbon:
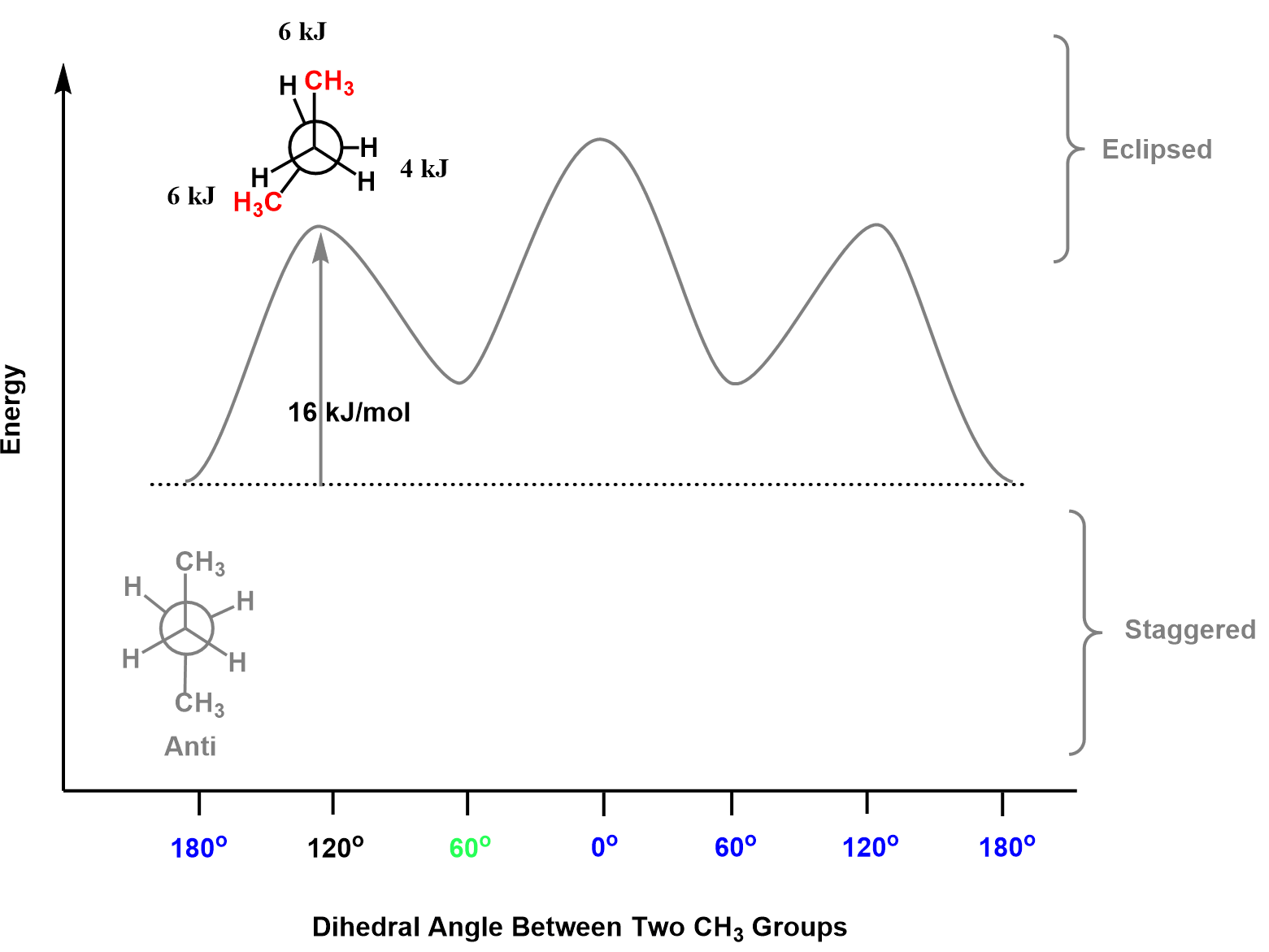
So how much higher in energy is this conformation compared to the ani? There are two CH3:H and one H:H eclipsing interactions, and from the table, we find the total energy of those to be:
2 x 6 kJ/mol + 4 kJ/mol = 16 kJ/mol
Therefore, this conformation is 16 kJ/mol higher in the energy diagram.
Another rotation by 60o gives a staggered conformation, and what is important here is that the two methyl groups are at 60o and this is called a gauche conformation. So, remember, depending on the dihedral angle between the two larger groups, the staggered conformation can be Anti (180o) or Gauche (60o).

It has been shown that in the gauche conformation, there are steric interactions between the methyl groups associated with 3.8 kJ/mol of destabilization. Therefore, the gauche conformation appears 3.8 kJ/mol higher than the anti conformation:
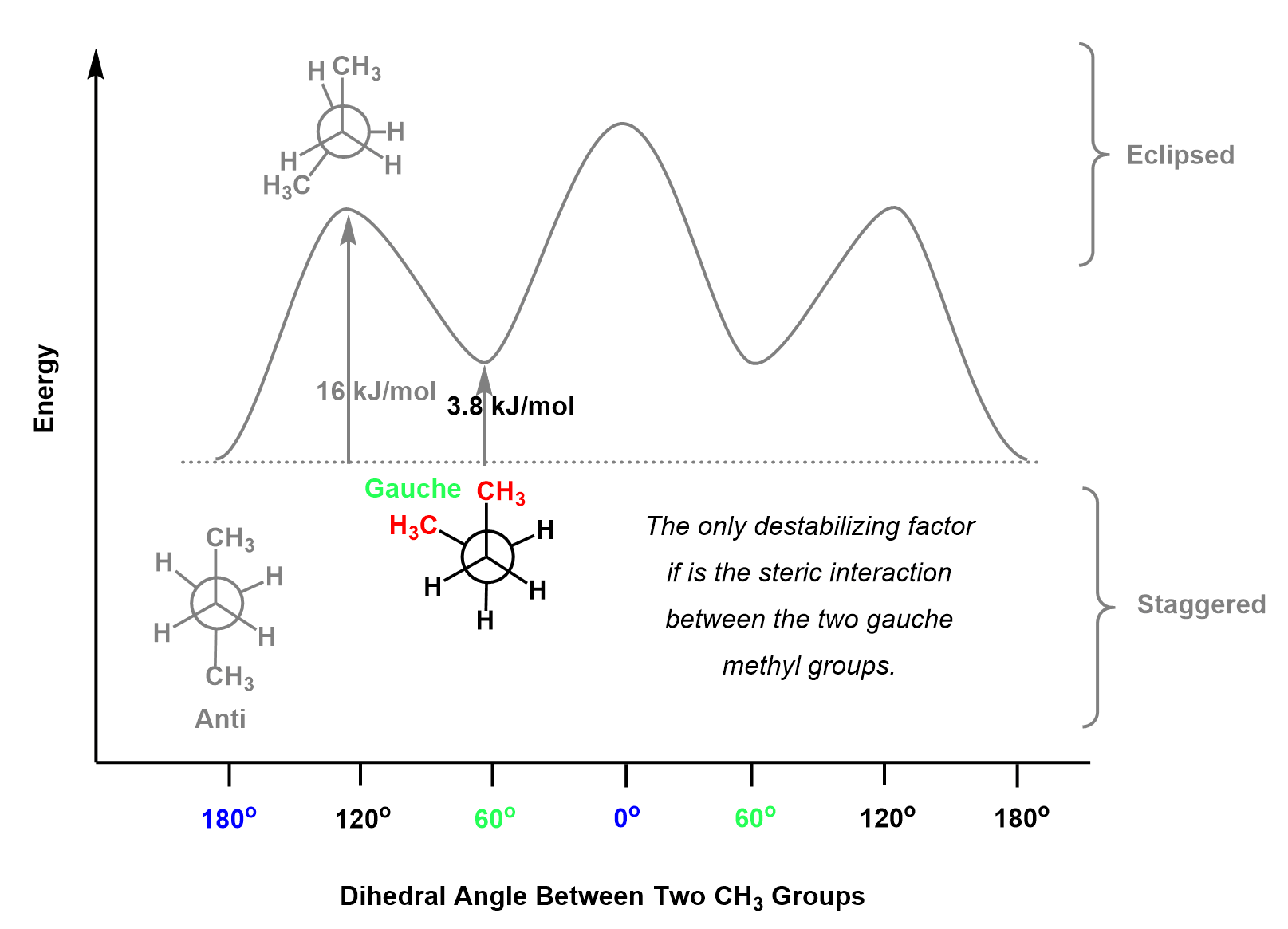
The next conformation is eclipsed and it is the highest in energy (least stable) because the two largest groups are facing each other which is sterically very unfavorable. The steric interaction between the methyl groups is largely due to the hydrogens getting to close as demonstrated in this video clip:
Remember also that when aligned, there is torsional interaction due to the repulsion between the bonding electrons. The staggered conformations, on the other hand, get some additional stability due to the hyperconjugation between the electrons in the bonding orbital and the empty antibonding orbital of the neighboring group. This illustration is from the post about the eclipsed and staggered conformations of ethane:

Going back to the energy of the syn conformation, it has been shown that total energy of two eclipsing methyl groups accounts for 11 kJ/mol of destabilization. In addition to this, there are also two pairs of eclipsing hydrogens, and the total energy gap compared to the anti conformation would be 19 kJ/mol:
11 kJ/mol + 2 x 4 kJ/mol = 19 kJ/mol
Therefore, this particular eclipsed conformation, called syn conformation, goes to the top of the energy diagram as the most unstable:
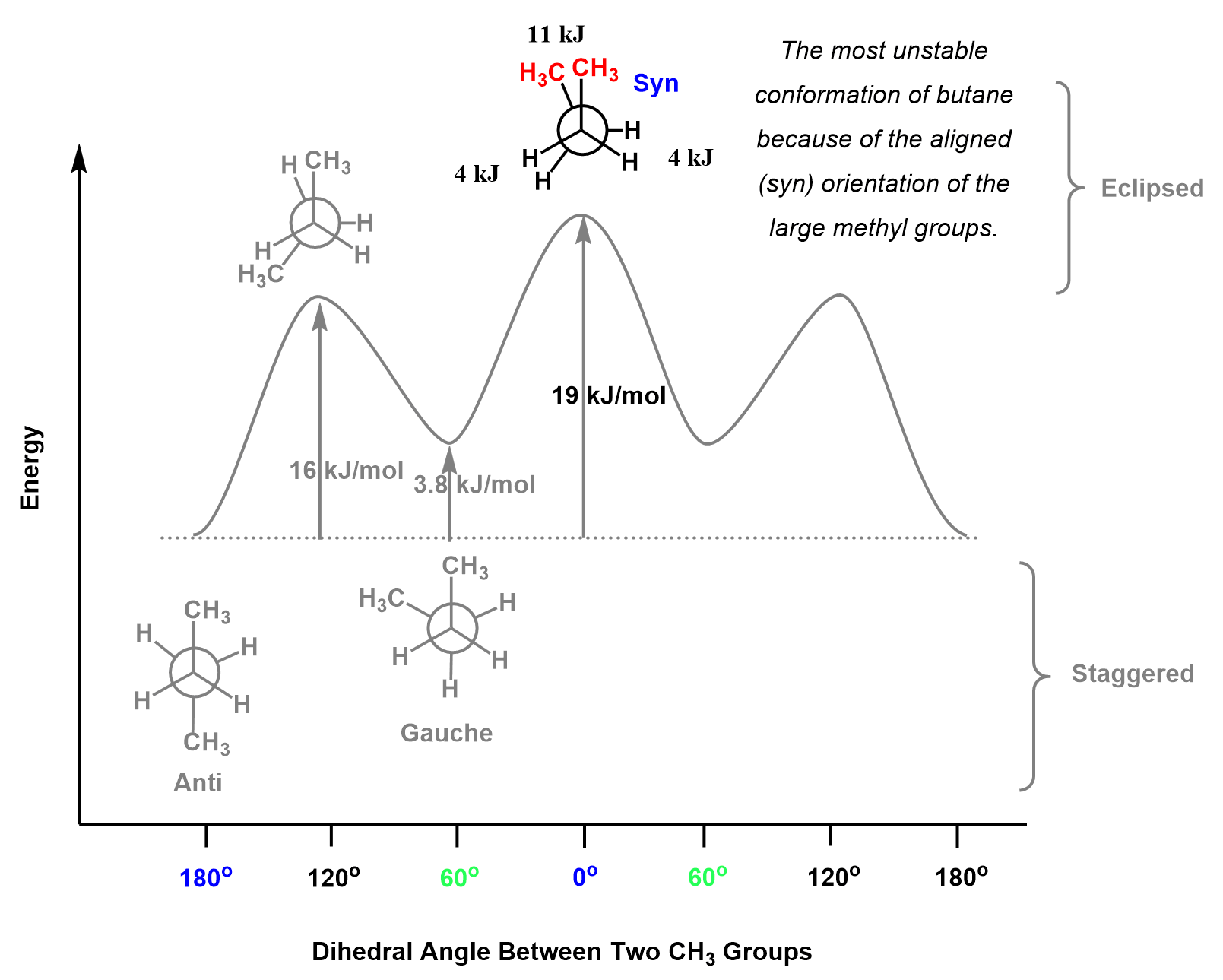
After this, the conformations are repeated, and we put them on same energy level as the previous one. For example, the 60o rotation of the back carbon from the syn conformation, gives a gauche conformation which is 3.8 kJ/mol higher that the anti conformation.
Side note: Only for those who have covered stereochemistry and enantiomers in particular. You probably do not need this, but for those of you who covered stereochemistry and wondering if the two gauche conformations are identical or enantiomers, the answer is they non-superimposable mirror images, and if we could freeze them in those conformations, they would be chiral. However, they rotations occur too fast and butane is achiral as it is optically inactive.
The subsequent 60o rotations give eclipsed and staggered (also anti because of the opposite orientation of the CH3 groups) conformations which we place on the energy diagram as discussed above:

In the end, we also summarize the arrangement and energy of butane’s conformations in a short video clip:
Check Also
- Naming Alkanes by IUPAC nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary Secondary and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
