In this post, we will talk about the ortho-, meta, and para directors in electrophilic aromatic substitution (EAS). As a reminder, the ortho-, meta, and para are the relative positions of the two groups in a disubstituted aromatic ring:

Let’s suppose we have a benzene ring with a substituent X. In electrophilic aromatic substitutions, the second substituent goes either to the ortho/para or the meta position of group X:

Here is the short answer to ‘How do I know if the electrophile will go to ortho, para, or meta position?’
Any Activating group directs the electrophile to the ortho and para positions.
Any deactivating group directs the electrophile to the meta position.

An activated ring means it undergoes an electrophilic aromatic substitution faster than benzene, and deactivated rings react more slowly than benzene.
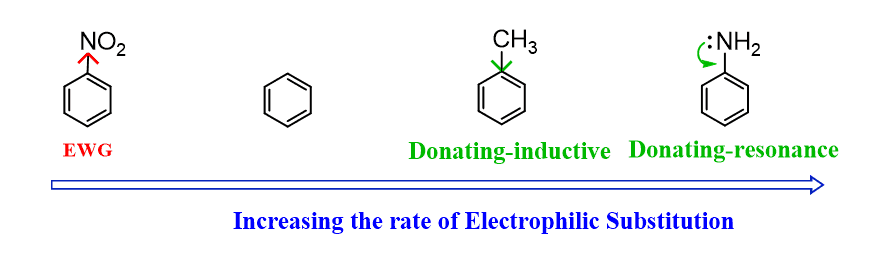
The activation and deactivation of the aromatic ring are caused by inductive or resonance effects (or both).
The inductive effect is a result of the different electronegativities of the carbon in the ring and the atom connected to it. There can be electron-donating (activating) and electron-withdrawing (deactivating) groups. For example, a methyl group activates the ring since the carbon is connected to three hydrogen atoms, and being more electronegative, it pulls the electron density and donates to the ring. And, in general, any alkyl group is an activator.
Halogens, on the other hand, are more electronegative than carbon, and when connected to the ring, they pull the electron density by the inductive effect and thus reduce its reactivity.
If the atom connected to the aromatic ring has a lone pair of electrons, then the ring is activated by the resonance effect, which is generally a stronger contributor:
The following table summarizes the activating and deactivating groups and their directing effect in electrophilic aromatic substitution reactions:

You can check this post for more details about the activating and deactivating groups. For now, let’s see how the activation and ortho, para regioselectivity work by the inductive and resonance effects.
Ortho Para Directors
In toluene, the ring is activated by an inductive effect since the methyl an electron-donating group, and electrophilic aromatic substitutions of toluene produce the ortho and para products in great excess compared to the meta product:
To explain this regioselectivity, we need to draw the complete electrophilic substitution mechanism, including all the resonance structures of the sigma complex:

Do you use any advantage of the ortho and para-substituted resonance structures? Think about the stability of carbocations.
In both ortho and para substitutions, there is a resonance structure with a tertiary carbocation stabilized by stronger hyperconjugation. This resonance contributor stabilizes the transition state, thus making the ortho and para substitution faster than meta substitution.
And this is true for any activating group. They all stabilize the transition state, making the reaction faster, directing the electrophile to the ortho, para positions.
Let’s now look at an example where the aromatic ring is activated by the resonance effect. For example, anisole is an activated aromatic ring and does not even require a catalyst to be halogenated because the resonance effect is very strong. In addition, halogenations are the fastest among all the electrophilic aromatic substitution reactions:
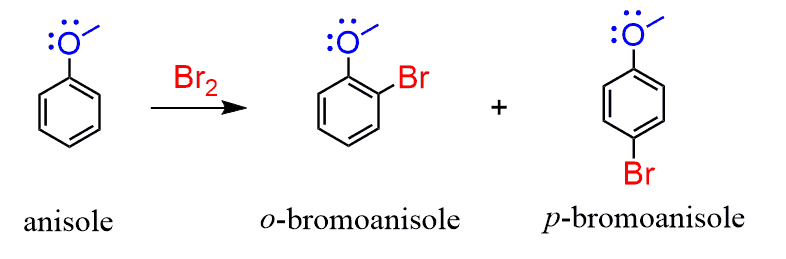
To explain the regioselectivity, draw all the resonance structures of the sigma complex:
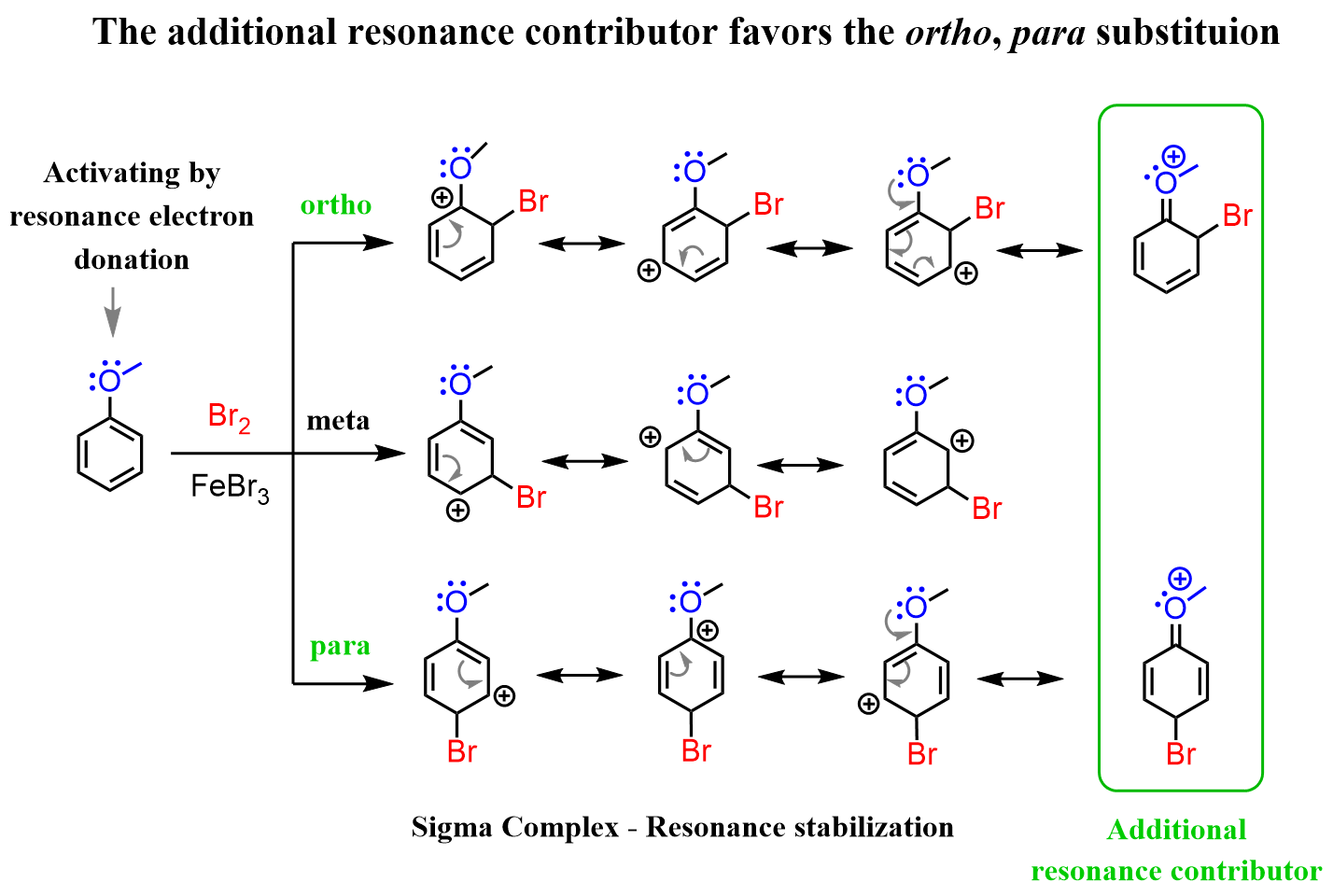
And what we see is that in this case, the ortho and para substitutions have an extra resonance structure involving the lone pair of the oxygen. This is a significant stabilization of the transition state since all the atoms have complete octets (check the stability of resonance structures).
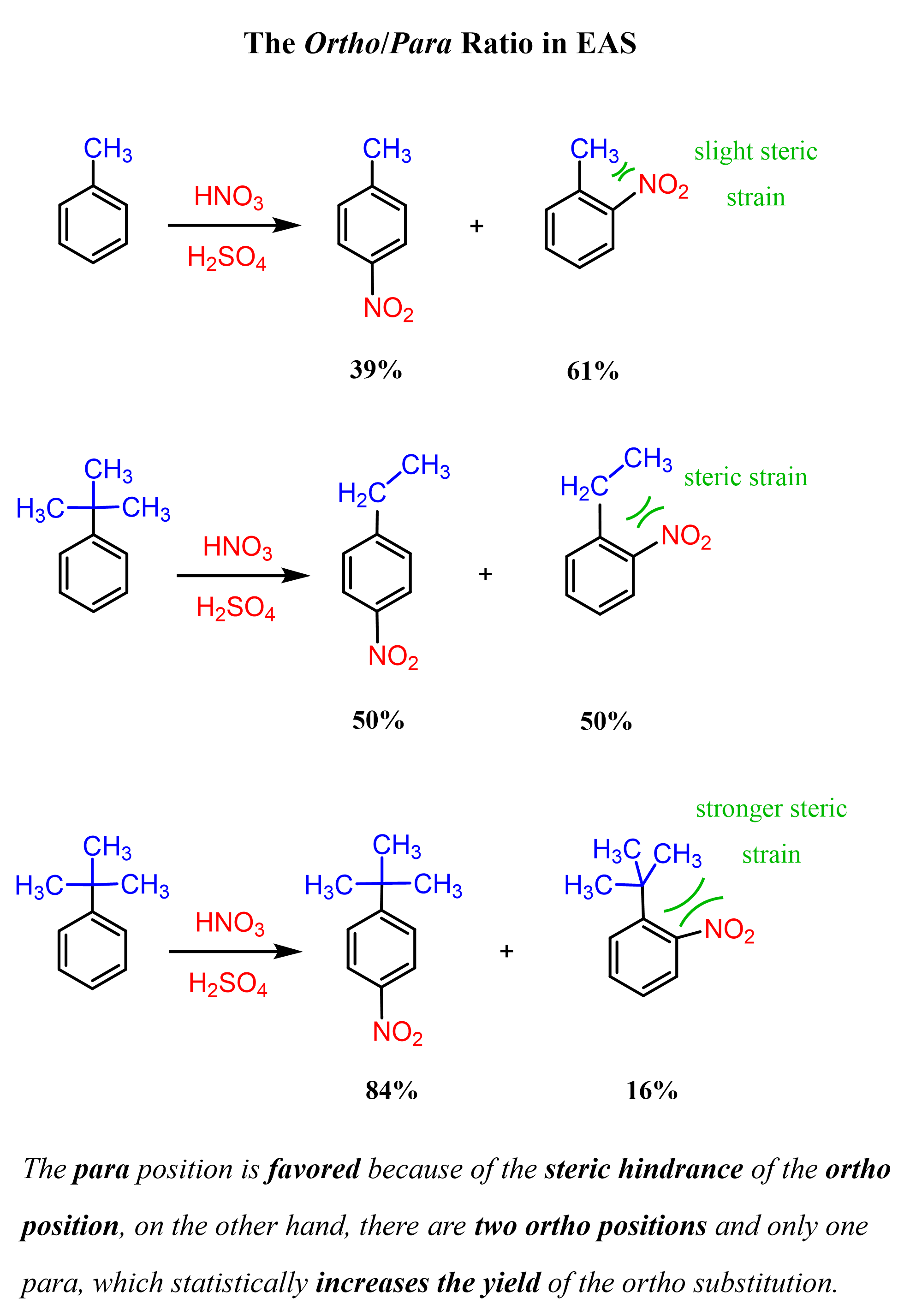
The Ortho–Para Ratio
The ratio of the ortho and para substitution is difficult to predict. On one hand, the para position is favored because of the steric hindrance of the ortho position; on the other hand, there are two ortho positions and only one para, which statistically increases the yield of the ortho substitution. The larger the group present on the ring, the more favored the para substitution would be. For example, the nitration of toluene (methyl benzene) produces a mixture of ortho and para isomers in approximately 60 : 40 ratio. Having a larger alkyl group increases the ratio in favor of the para isomer because of greater steric hindrance:
A similar pattern can be seen when comparing the ortho/para ratio for the nitration of phenol and anisole. In this case, though, the intermolecular interaction, such as hydrogen bonding between the OH and NO2 groups, also contributes to the formation of the ortho isomer:
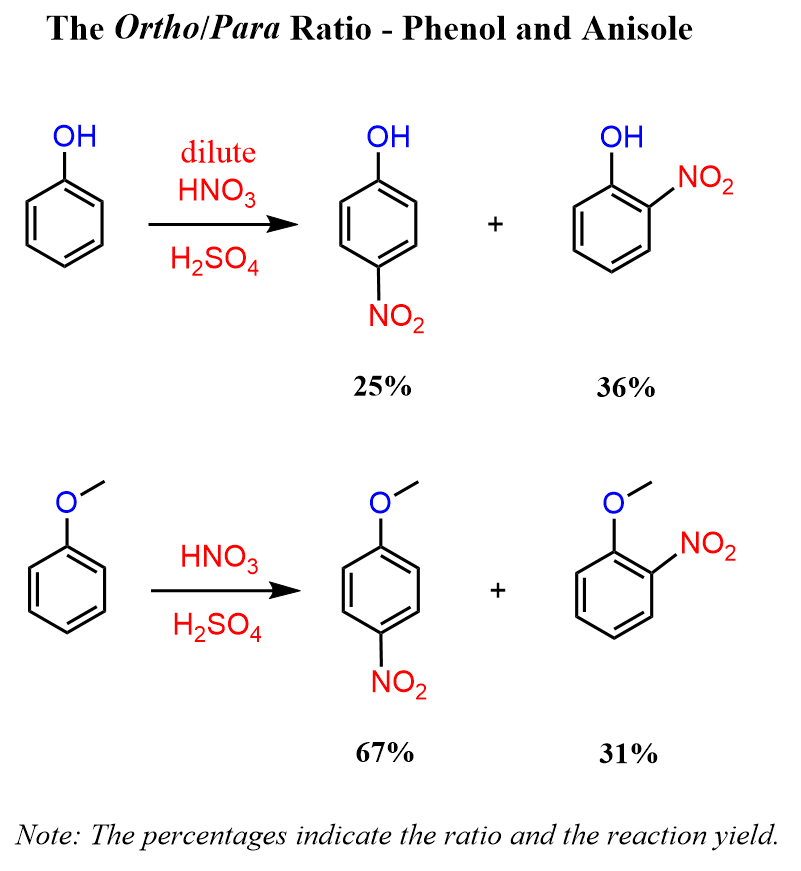
Tetrahedron, Volume 55, Issue 22, 28 May 1999, Pages 6733-6738
Notice also that for the nitration of phenol, dilute nitric acid is used to avoid undesired oxidation reactions.
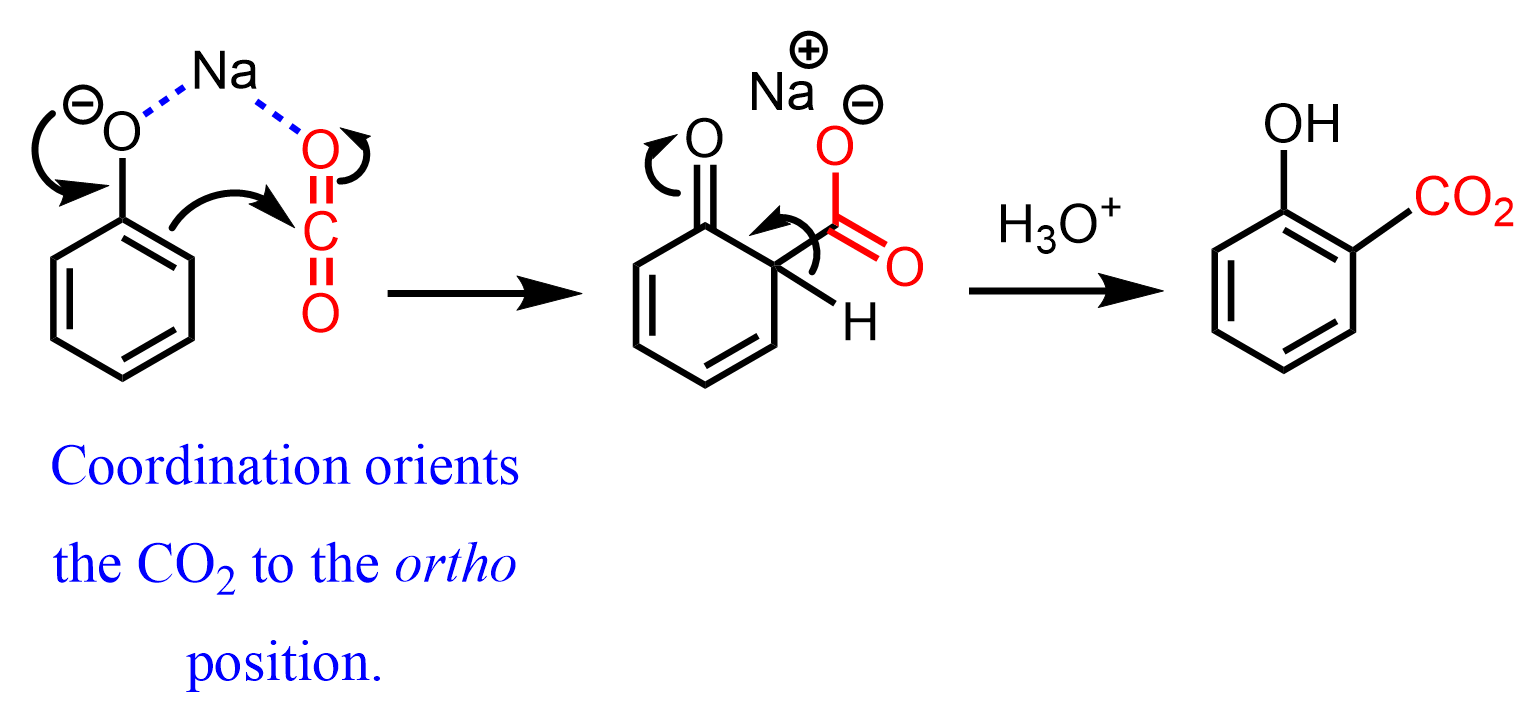
Intermolecular interactions are also responsible for the ortho-carboxylation of phenol in the synthesis of aspirin.
Sometimes, activating groups do not give the expected ortho, para-substitution. For example, even though the amino group activates the benzene ring, and some electrophilic aromatic substitution reactions of aniline are faster compared to those with benzene, nitration of aniline leads to a mixture of undesirable side products, or, what is interesting, meta-nitroaniline is obtained:
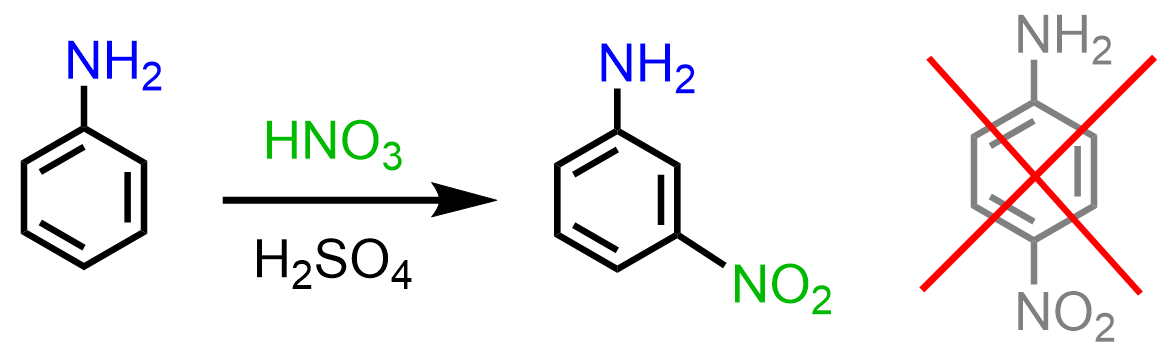
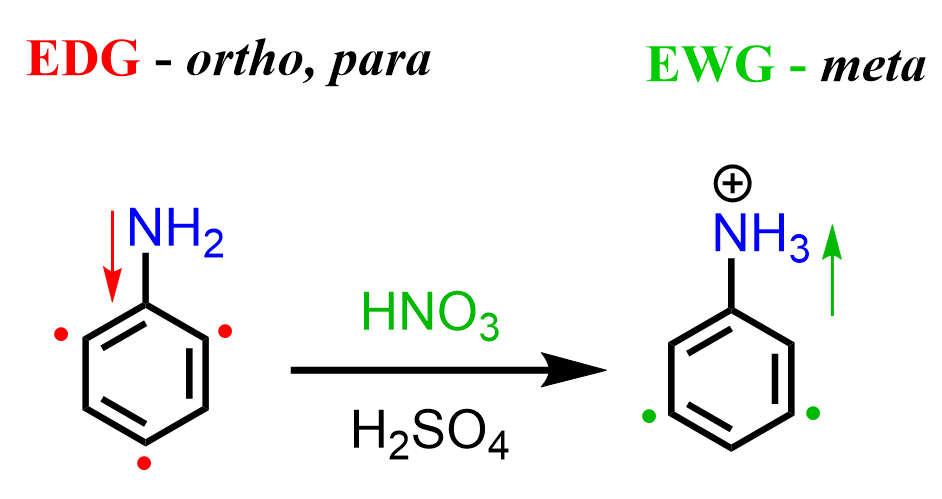
The formation of the unexpected meta product is due to the basicity of the amino group. While in Friedel Crafts reactions, it acts as a Lewis base; here, we have a regular Bronsted acid-base reaction with HNO3 and H2SO4. Once the amino group protonates, a strongly deactivating -NH3+ group is formed, which now undergoes a meta EAS:
In this case, as well, the strategy is to convert the amino group to an amide and hydrolyze it back to amine once the nitration is complete.
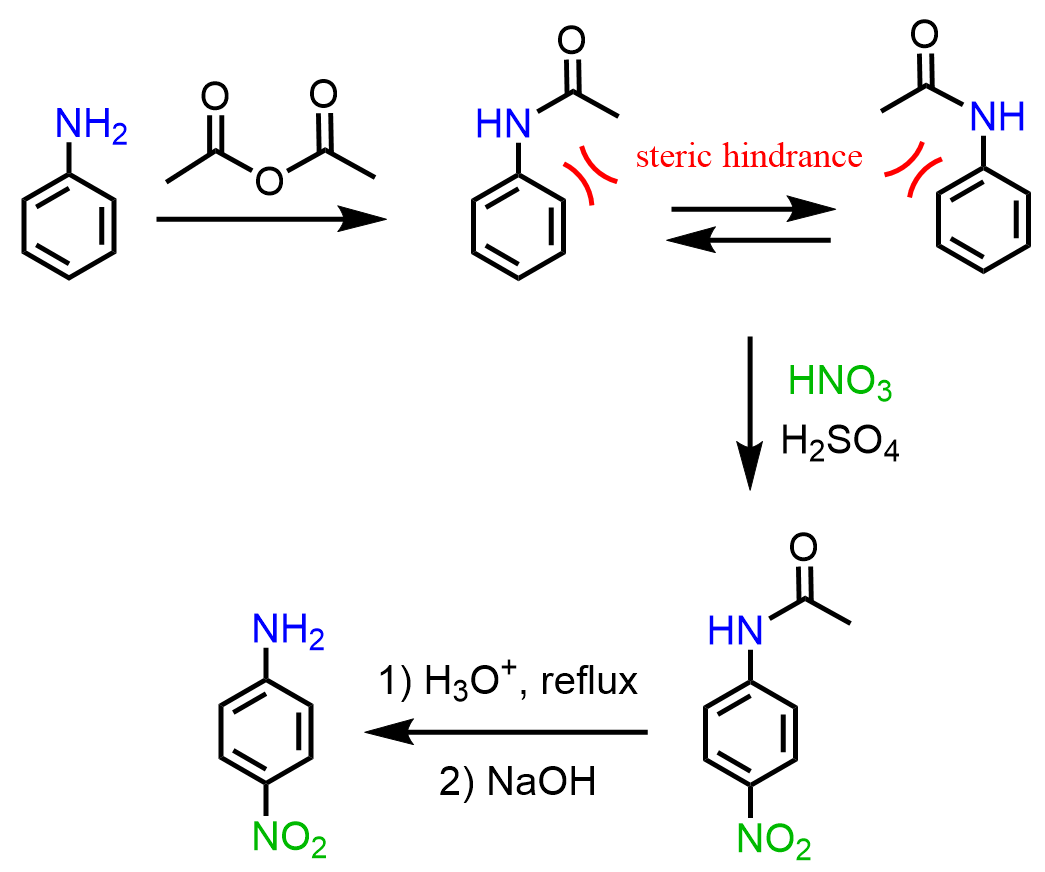
Interestingly, it was found that, unlike nitration, aniline can be submitted to direct sulfonation using concentrated sulfuric acid at 190 oC, and the para isomer is obtained (Clayden – Organic Chemistry):
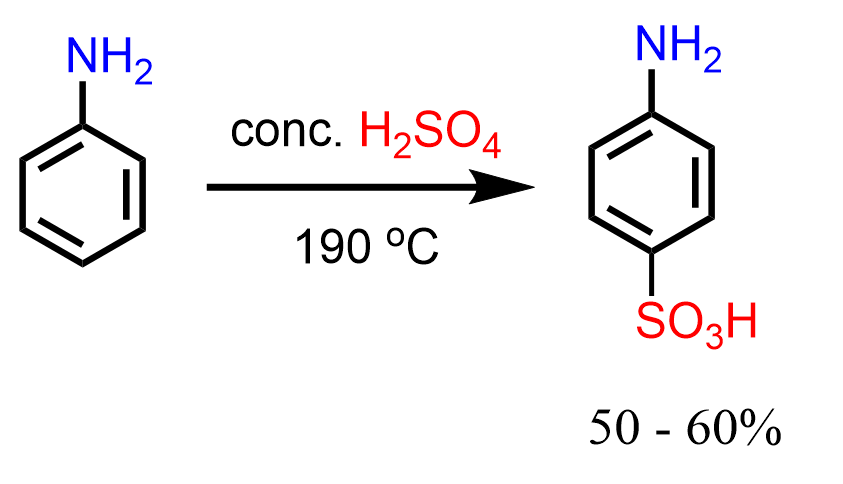
One explanation for this is the superior reactivity of the trace amount of the unprotonated aniline. You can find more about exceptions and limitations of electrophilic aromatic substitution in this article.
Meta Directors
Deactivating groups slow down electrophilic aromatic substitutions and direct the substituents to the meta position. The only exceptions are the halogens that deactivate the aromatic ring, yet direct the electrophile to the ortho and para positions. There is a separate post about this, which you can check here.
Let’s explain why the nitration of nitrobenzene occurs mainly at the meta position:
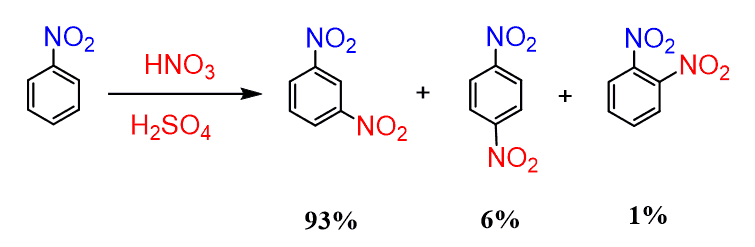
As usual, go ahead and draw the complete mechanism with the resonance structures. We will use E instead of NO2 as a general mechanism:

When we discussed the regioselectivity of activated aromatic rings, we saw the advantage of ortho and para-substituted carbocations being better stabilized either by inductive or resonance effects. In this case, it is more like the disadvantage of the ortho and para resonance structures that makes the meta substitution a preferred pathway for aromatic substitution. Both in ortho and para, there is a resonance structure where the positively charged carbon is next to the electron-withdrawing nitro group, which makes the transition state more unstable.
The carboxyl group, trihalogenated alkyl groups, and positively charged amino groups are also electron-withdrawing groups and act in a similar way.
So, electron-withdrawing groups slow down substitutions at all the positions, but they do it less for the meta substitution. They favor the meta substitution only because it is less unfavorable than the ortho and para substitutions.
In the following practice problems, we will go over the ortho-, para-, and meta – directors in Electrophilic Aromatic Substitution. The first exercise is to make sure you know which groups are activators and deactivators, as well as how that relates to the Ortho-, Para-, and Meta – directing effects.
The second practice problem is built on this and gives specific examples where you need to predict the major product of each Electrophilic Aromatic Substitution reaction.
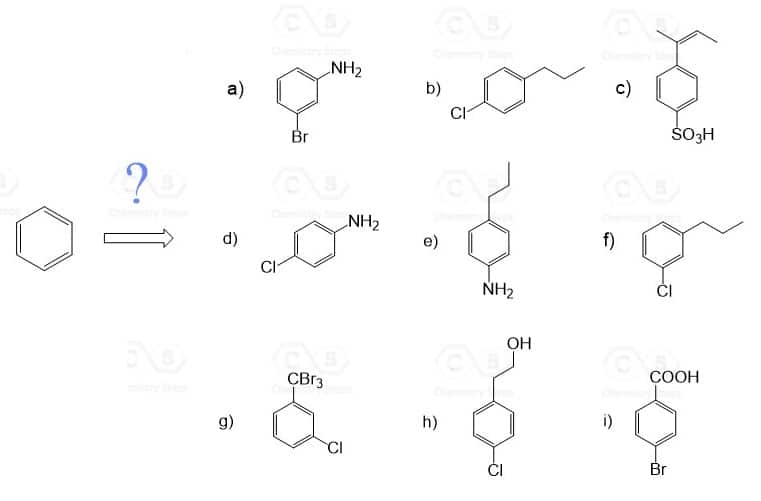
In the third practice exercise, we will go over the Synthesis of Disubstituted Benzenes. The order of introducing each group on the benzene is crucial here as it dictates whether the next group will be placed in ortho, para– or meta position.


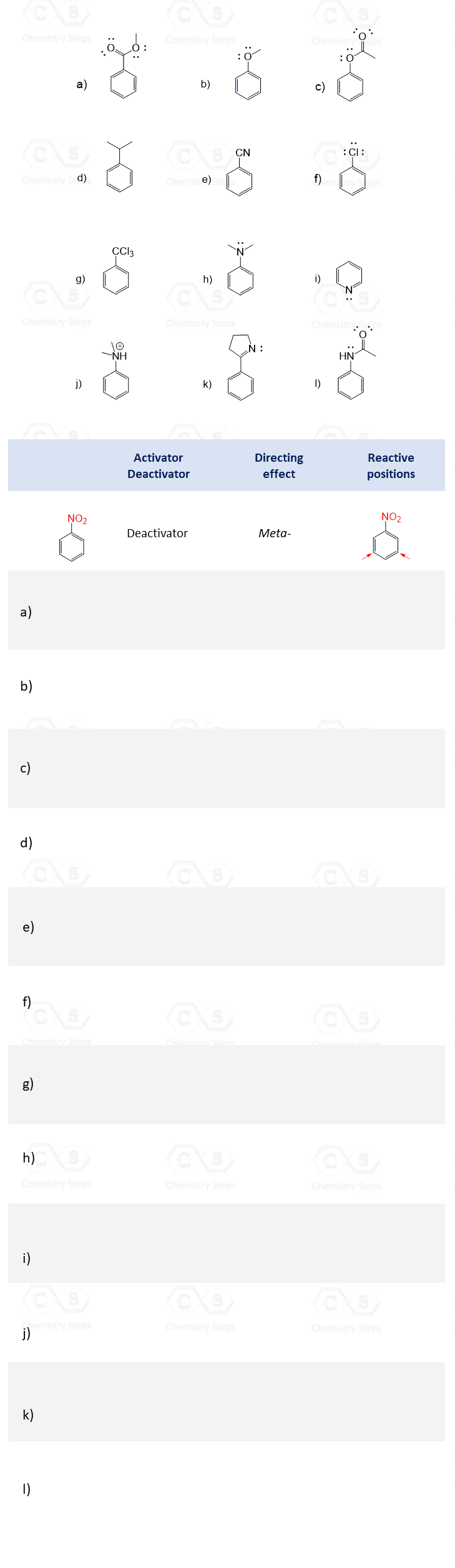

The answer to question F in Part 3 does not match the question. What about the ketone reduction?
Thank you for pointing it out. Fixed.