By experimental methods, which we’ll discuss later, it has been shown that cycloalkanes are generally less stable than their corresponding alkanes. More accurately, the comparison was made between a cycloalkane and the equivalent number of CH2 groups. Recall that cycloalkanes have a general formula of (CH2 or CnH2n) while alkanes have the CnH2n+2 formula, and therefore, the direct comparison wouldn’t be quite fair.
This instability of cycloalkanes is explained by what is called a ring strain. The ring strain is the combination of two destabilizing factors: angle strain and torsional strain.

Let’s start with the angle strain.
Angle Strain
To understand the bond angle strain, we need to first recall that cycloalkanes are composed of saturated carbon atoms, which means they are sp3-hybridized with tetrahedral geometry, and the ideal bond angle should be 109.5o. Making a ring, composed of atoms with a set bond angle of 109.5o, raises tension as it requires changing this angle. The more it is changed, the higher the tension, thus the bond angle strain. Below are the ideal bond angles for cycloalkanes if they were flat – all the carbon atoms in one plane:
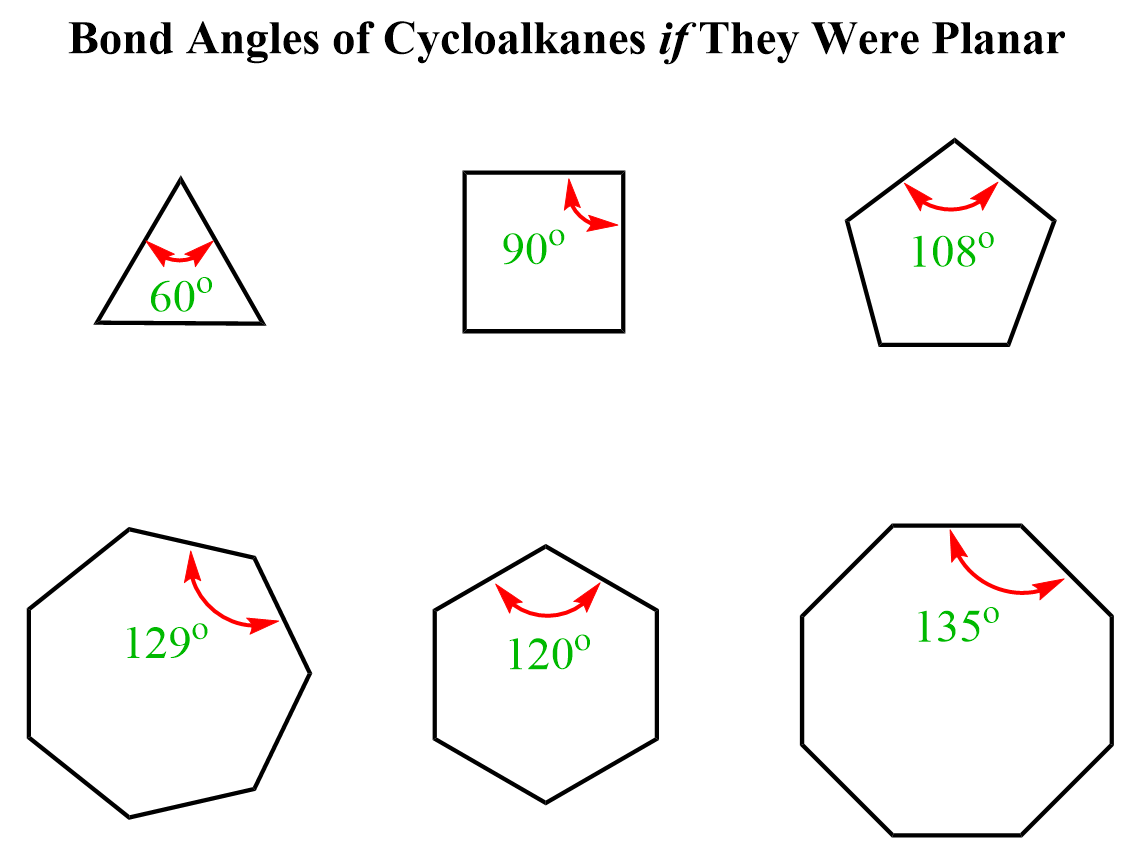
Recall that we can calculate the angle using this formula, where n is the number of atoms in the ring:
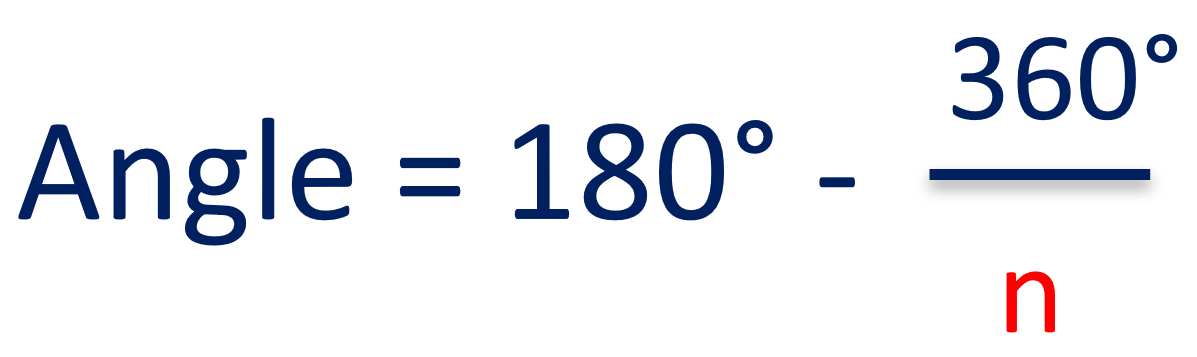
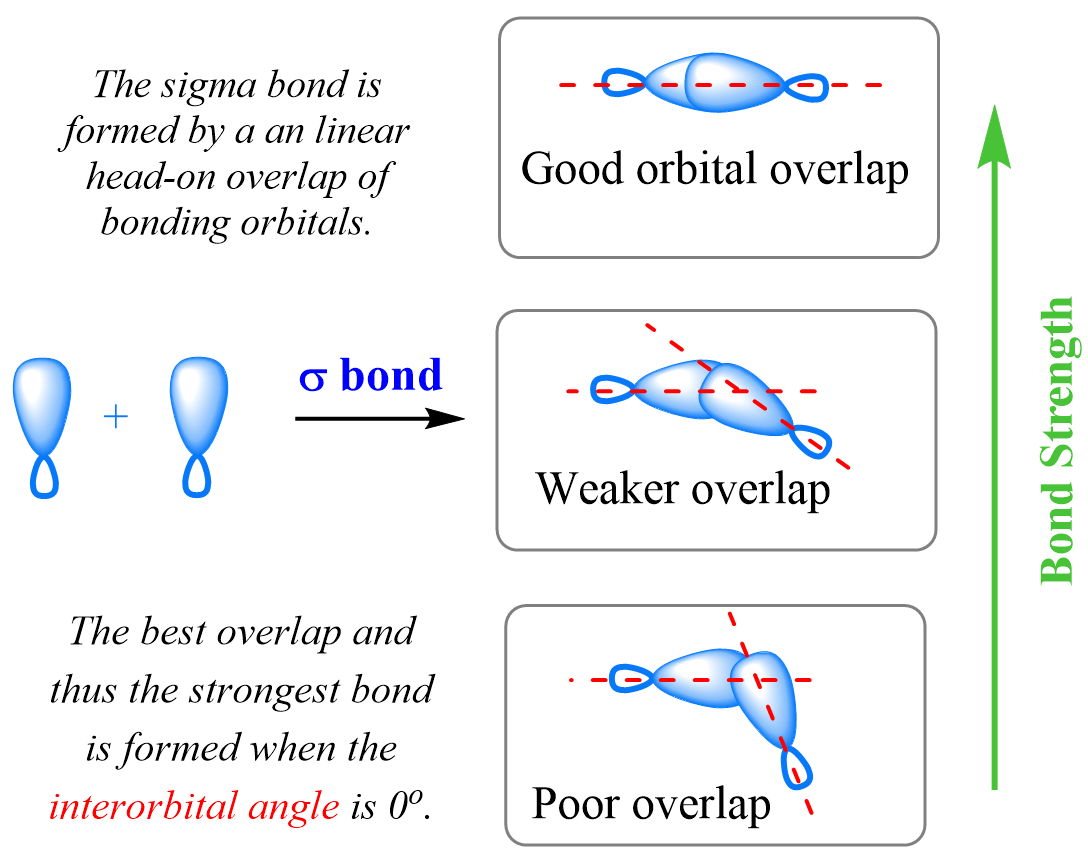
The deviation from the ideal bond angle causes a poor overlap of the orbitals, making the C-C sigma bond, which explains the bond angle strain. Recall that sigma bonds are formed by linear head-on overlap of the bonding orbitals, which is impossible for cyclopropane and cyclobutene because the angle between the carbon atoms is far smaller than 109.5o:
The larger this deviation from the ideal bond angle, the greater angle strain and thus instability it brings to the molecule. For example, in cyclopropane, the difference from the ideal bond angle is 109.5o – 60o = 49.5o °, and it is the least stable among the cycloalkanes.
Notice that the angle between the atoms and the bond orbitals is not necessarily the same. In cyclopropane, the angle between the carbon atoms is 60o while the interorbital angle has been shown to be 104o:
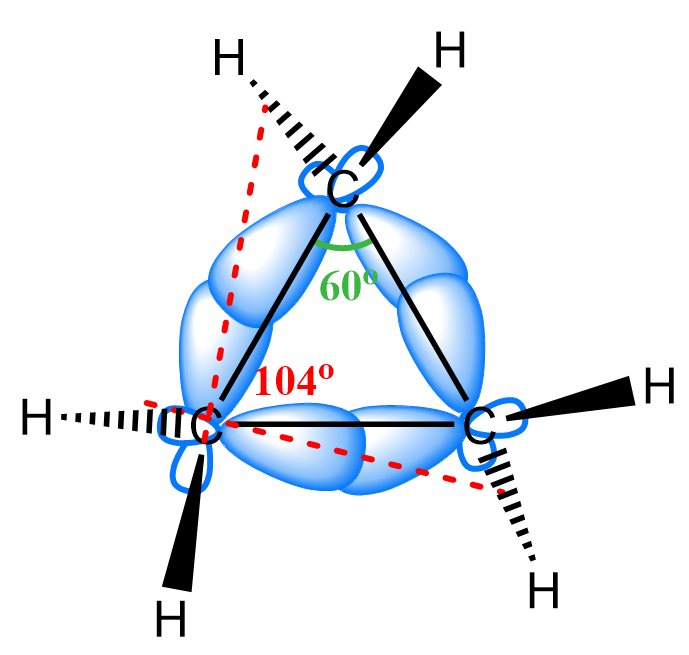
It is a matter of debate whether the carbon atoms are sp3 or sp2-hybridized, and one supporting argument of sp2 hybridization is the fact that cyclopropane can be hydrogenated like alkenes are.
The expected deviation from the ideal bond angle in cyclobutene would be 109.5o – 90o = 29.5o which is smaller than in cyclopropane. Aside from this, as the rings get larger, they start getting the possibility of adopting different conformations to reduce the angle strain and the torsional strain as well. So, before getting to larger rings, let’s go over the torsional strain as well.
Torsional Strain
Torsional strain also known as the eclipsing strain is the result of repulsive dispersion forces that cannot be relieved due to restricted conformational mobility. Recall from the discussions about Newman projections and conformational analysis that the torsional strain is a type of steric hindrance due to small eclipsing groups – H/H, or H /CH3.
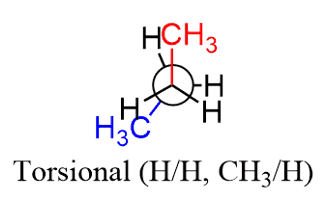
When two large groups are eclipsed, there is both torsional and steric strain:

The torsional strain is especially relevant in small cycloalkanes because of the restricted conformational freedom. Now that we know how the angle strain and torsional strain make up the overall ring strain, let’s see, in order, how they affect the conformation and stability of cycloalkanes.
Cyclopropane
This is the smallest of the cycloalkanes with the greatest ring strain and thus the greatest instability. In addition to the highly strained bond angles, the ring is, of necessity, planar, and all the C−H bonds are eclipsed, thus the pronounced instability of the molecule:
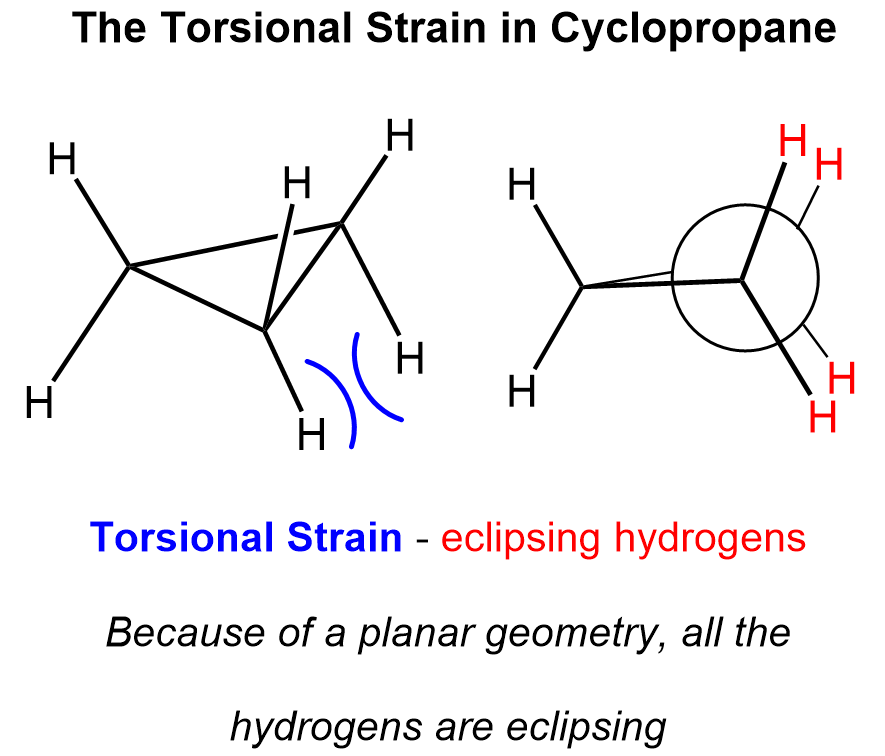
Because of this high torsional strain and the unfavorable bond angle which results in weak C-C bonds, cyclopropane can be hydrogenated to open up the ring using hydrogen in the presence of a palladium catalyst:
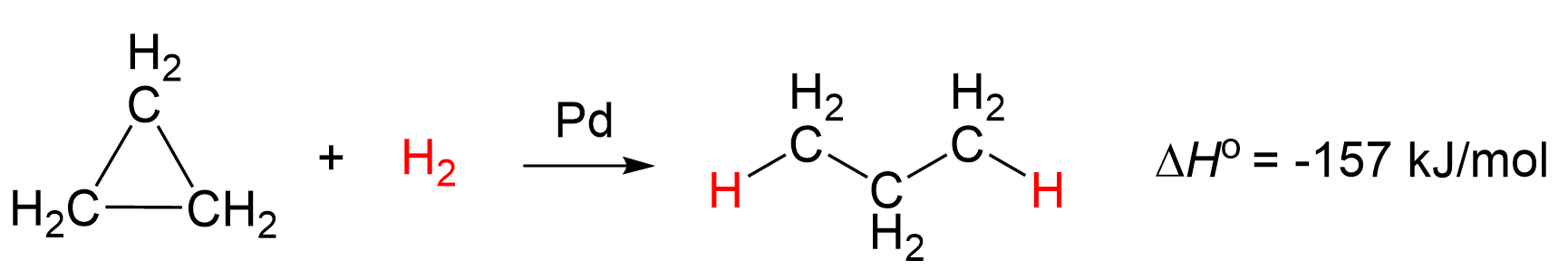
The bond dissociation energy of the C-C bonds in cyclopropane is 65 kcal/mol (272 kJ/mol), whereas in ethane, for example, it is 90 kcal/mol (377 kJ/mol).
Cyclobutane
The bond angles in cyclobutene would be 90o if it had a planar geometry like cyclopropane. The angle strain here wouldn’t be as severe; however, there would be eight eclipsing C-H bonds resulting in a greater torsional strain. Because of this, cyclobutene adopts a slightly bent geometry, also known as a puckered conformation, to reduce the torsional strain. The trade for this is the smaller bond angle (88o), which is still energetically more favorable than having all the C-H bonds eclipsing:
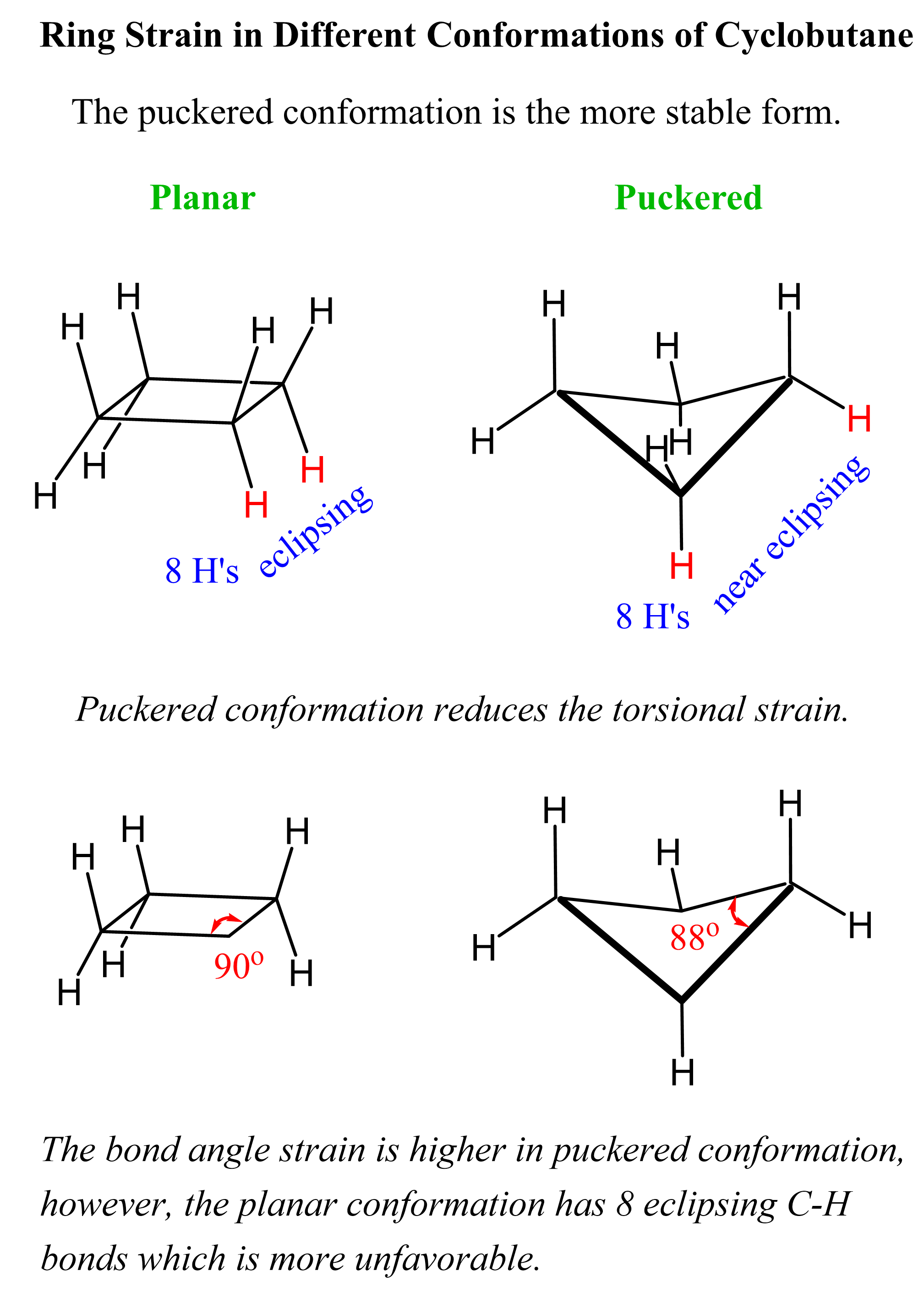
Because of the ring strain and the relatively poor overlap, the C–C bond strength in cyclobutane is 63 kcal/mol (264 kJ/mol), which is still lower than in regular alkanes. Itis less reactive than cyclopropane, but can still be hydrogenated in the presence of a Pd catalyst:
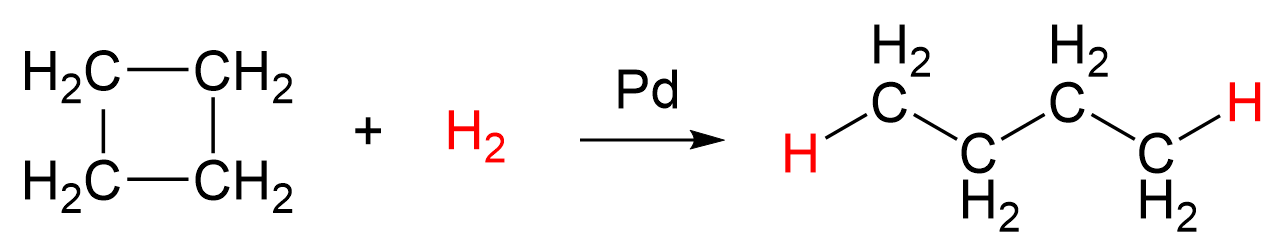
Cyclopentane
The bond angle of a pentagon is 108o which is very close to the tetrahedral angle of 109.5o. Cyclopentane, however, is not planar because that conformation would result in 10 eclipsing C-H bonds. And, like cyclobutene, it assumes a slightly bent conformation commonly known as envelope conformation:
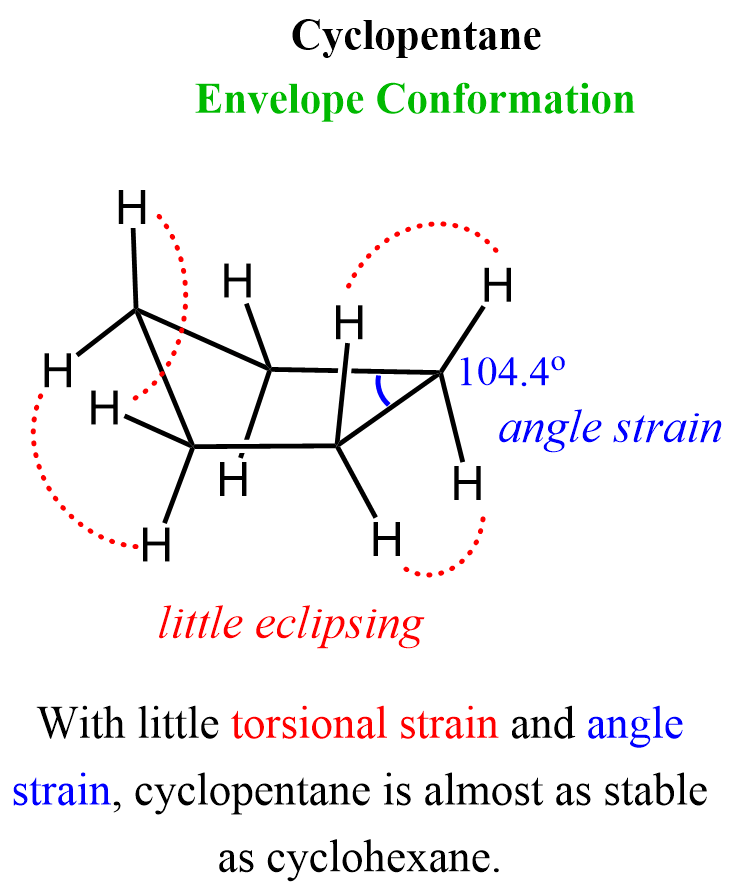
There is still a little torsional strain and angle strain, which make cyclopentane slightly less stable than cyclohexane. Neither of these has any unusual reactivity characteristics for cyclopropane and cyclobutene.
Cyclohexane
Cyclohexane is the most common ring structure found in many natural and synthetic structures. The main reason for this abundance is its great stability, as it lacks any angle and torsional strain. This is achieved by adopting the most stable conformation known as the chair conformation. The chair conformation puts the carbon atoms at nearly an ideal 109.5o tetrahedral angle (111.4o in some literature) and excludes any eclipsing hydrogen atoms:
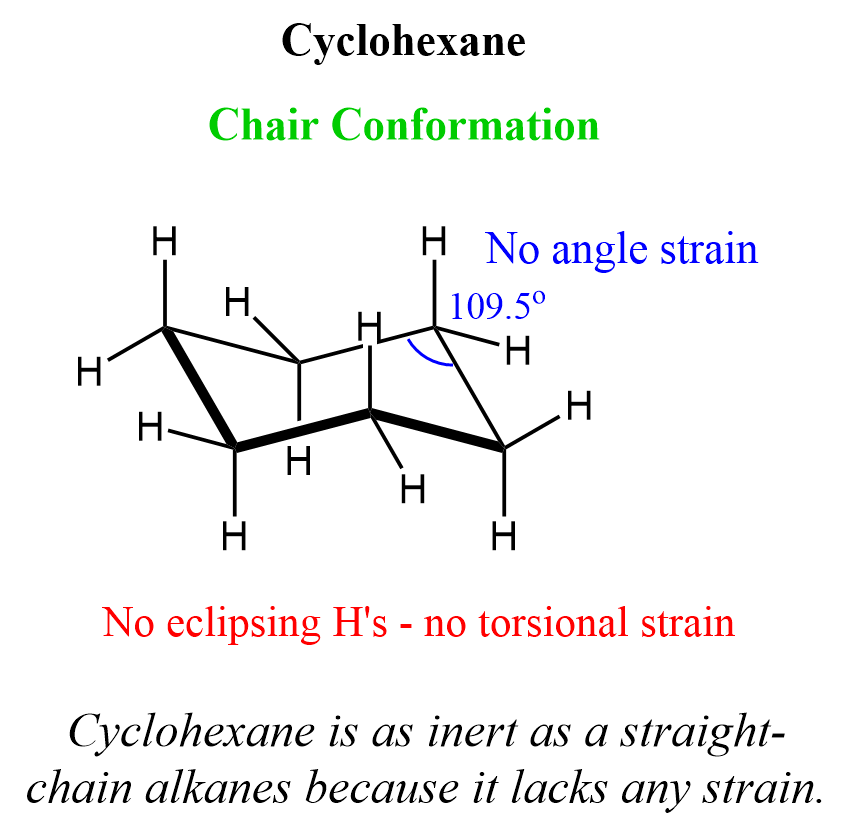
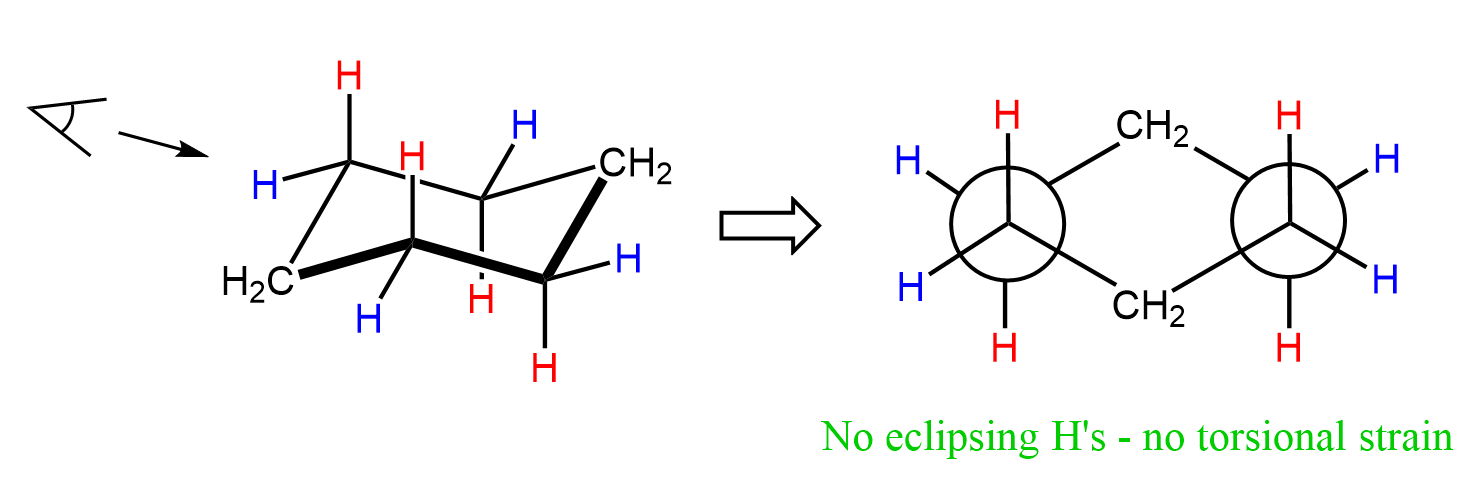
We can draw a Newman projection of cyclohexane in a chair conformation to confirm that the hydrogen atoms are maximally separated, thus lacking a torsional strain:
Cyclohexene undergoes an interconversion (ring flip) from one char conformation to another via rotation about the C-C single bonds:

The color-coded hydrogens do not imply the same atom. It is rather to show their position classified as axial and equatorial in each chair conformation.
Drawing the chair conformations of cyclohexane, and knowing the axial and equatorial positions is an essential skill you need to know in organic chemistry. We have separate articles for the ring flip, stability, and 1,3-diaxial interactions in substituted cyclohexenes, so feel free to check them out as well.
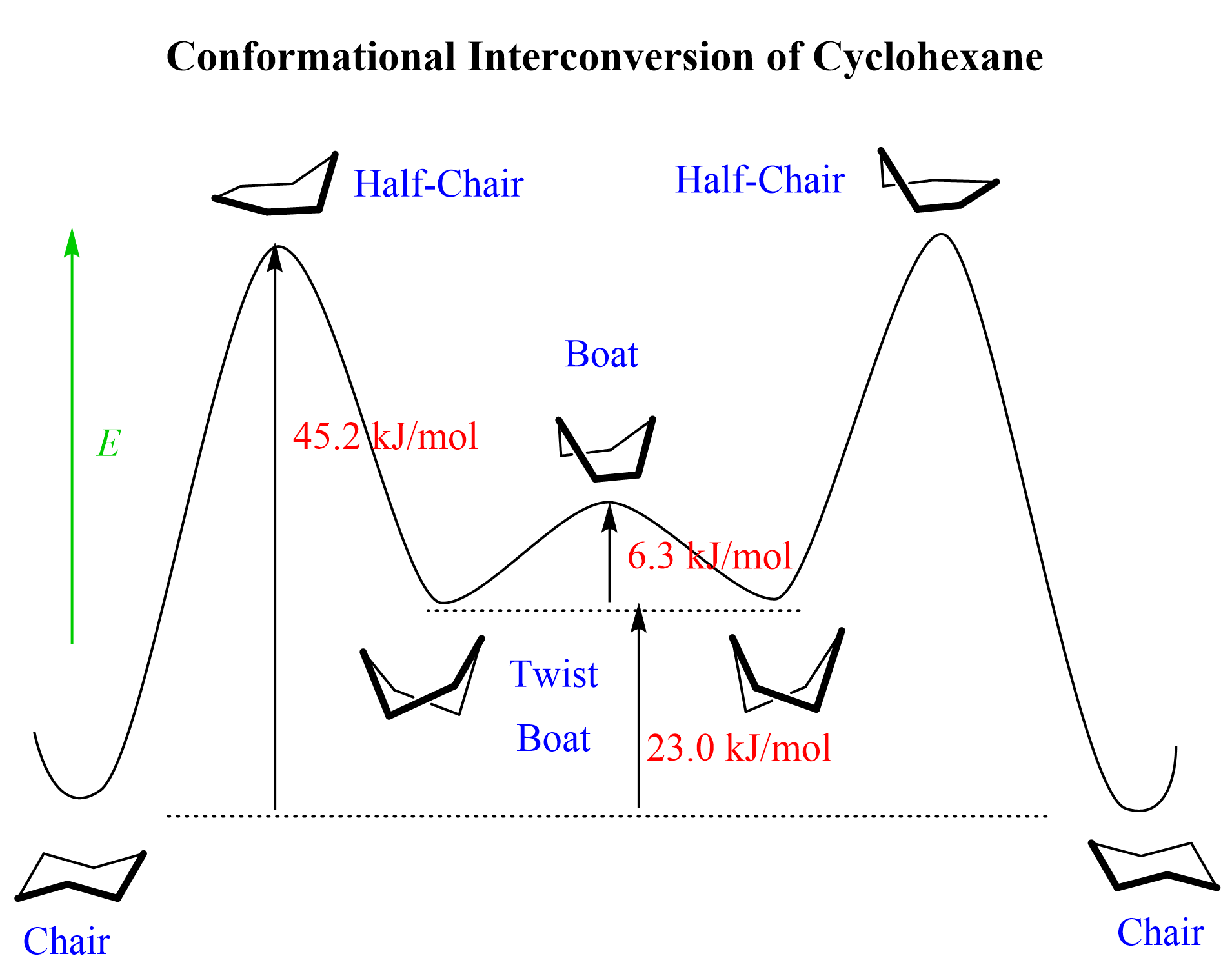
There are other, less stable, conformations of cyclohexane known as the boat, half boat, and twist boat, and the ring flip passes via higher energy barriers to adopt these conformations:
Heat of Combustion of Cycloalkanes
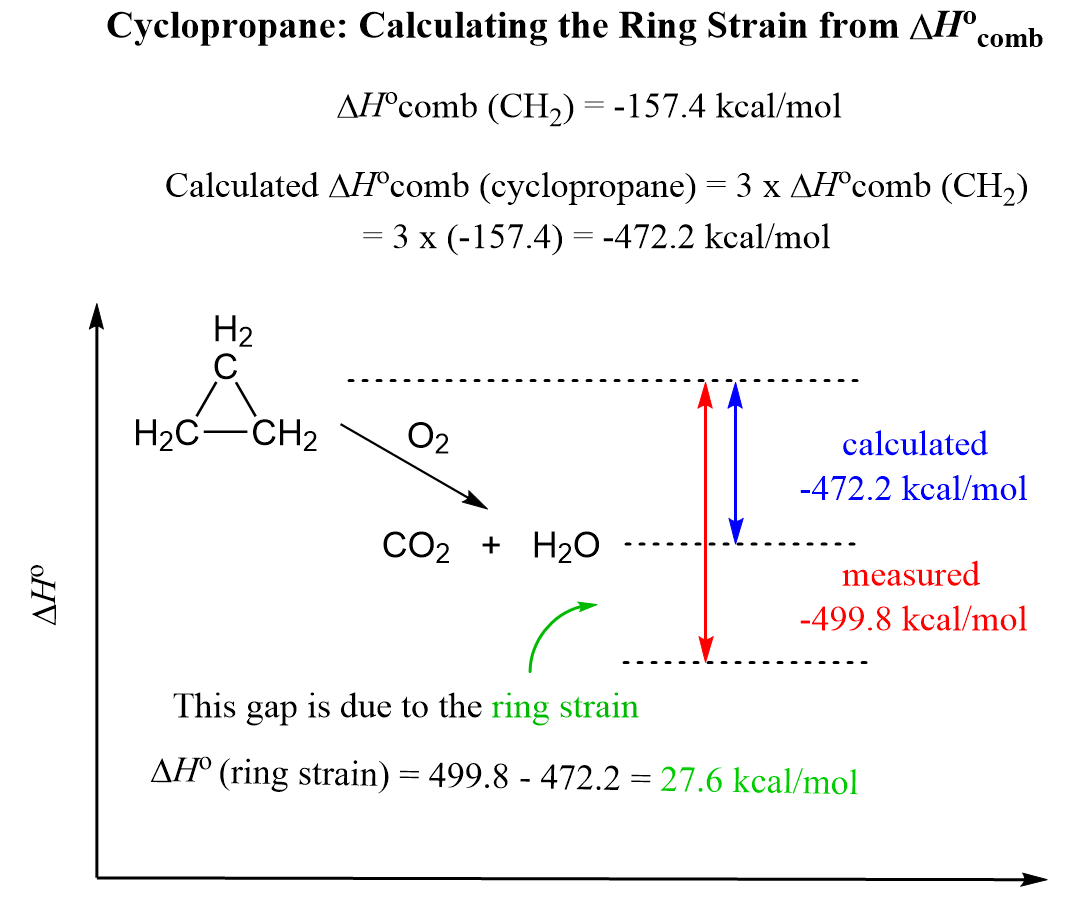
Let’s now discuss how the stability of cycloalkanes is determined. By comparing the enthalpy change for the combustion reaction of alkanes (ΔHocomb), it is estimated that the ΔHocomb for one CH2 unit is about -157.4 kcal/mol (-658.6 kJ/mol). Having this number, we can compare the ΔHocomb of each cycloalkane with the estimated value of ΔHocomb obtained by multiplying the ΔHocomb of the CH2 unit by the number of carbon atoms in the ring. For example, cyclopropane has three CH2 units, and therefore, we estimate that its ΔHocomb is three times that of ΔHocomb (CH2):
Calculated ΔHocomb (cyclopropane) = 3 x ΔHocomb (CH2) = 3 x (-157.4) = -472.2 kcal/mol
Experimentally, however, it has been determined that ΔHocomb (cyclopropane) is -499.8 kcal/mol. The difference in these values is the energy associated with the ring strain:
Ring strain = ΔHocomb (measured) – ΔHocomb (calculated)
Ring strain (cyclopropane) = 499.8 – 472.2 = 27.6 kcal/mol (115.5 kJ/mol)
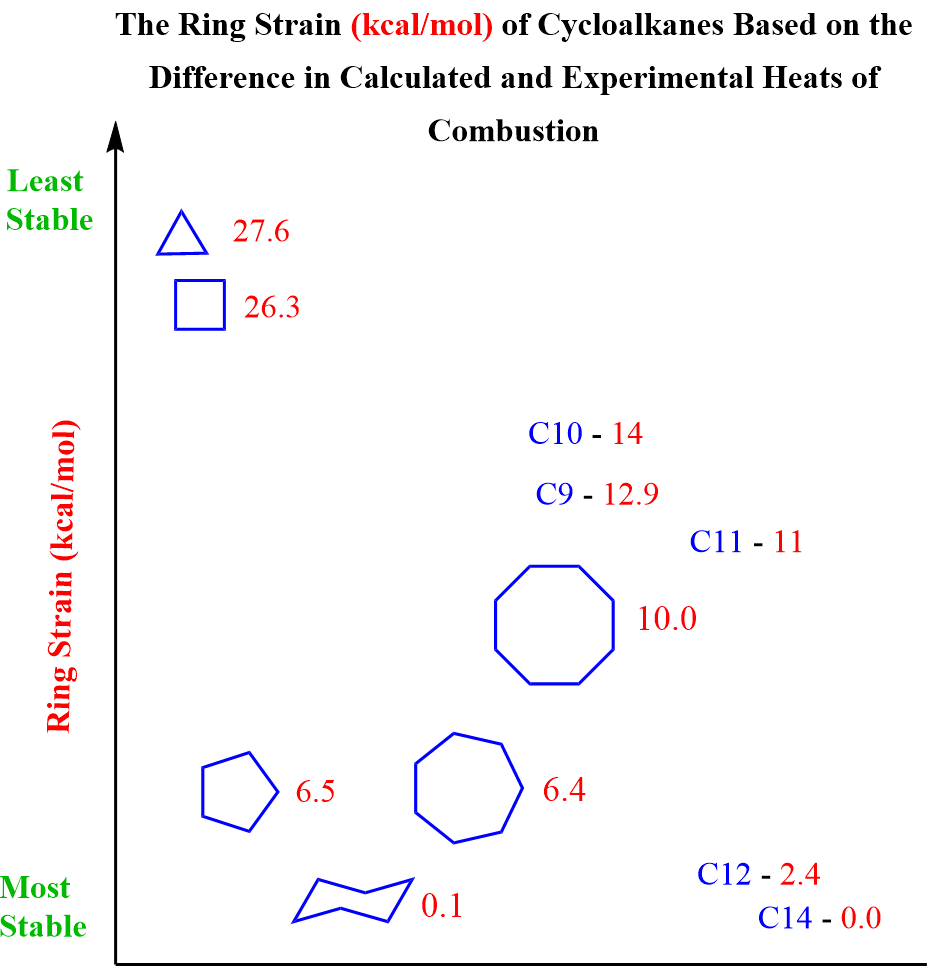
In similar experiments, the ring strain, or the absence thereof, was determined for other cycloalkanes, and the data are summarized in the following table:
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Newman Projection of Chair Conformation
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
