Back in general chemistry, you have encountered organic molecules which were shown either by a formula or a 2-D structure where all the atoms were shown in the same plane. For example, methane, ethane, propane, and even complex molecules such as glucose were shown with plane lines (bonds):

Now, one of the first and key differences between general and organic chemistries is that here we show molecules in 3-D representation. The 3-D structure of the molecule is as important as its formula, and you will see this during your organic chemistry 1 and 2, and even more advanced subjects such as biochemistry and medicinal chemistry.
Let’s compare these two representations of ethane:
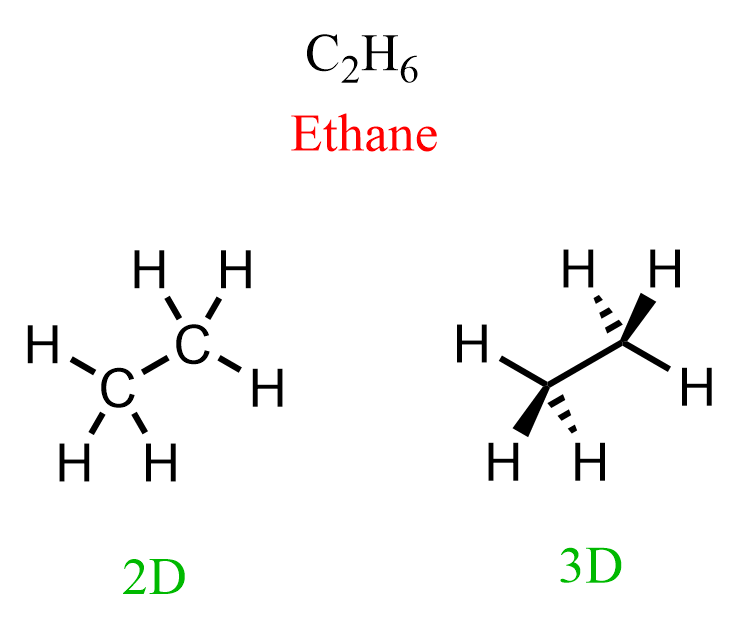
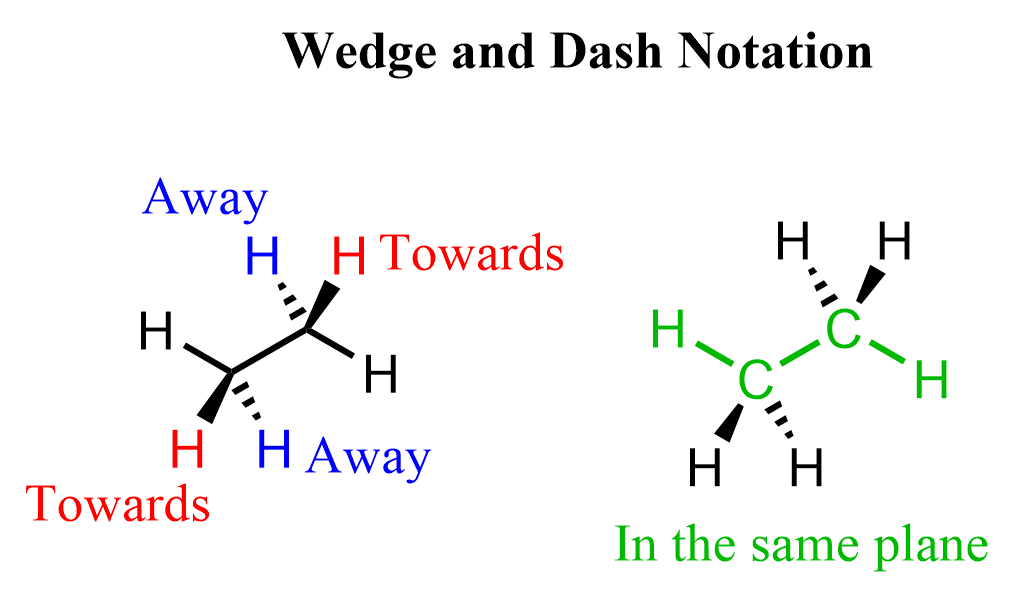
In the first one, we only show how the atoms are connected while in the second, we also emphasize the special arrangement of the atoms. You need to remember that carbon in alkanes is sp3 hybridized, and has a tetrahedral geometry. So, both carbons in ethane are tetrahedral, and when we put them in the plane of the paper, two hydrogens on each carbon are pointing towards us (wedge lines), and two of them are pointing away from us (dash lines):
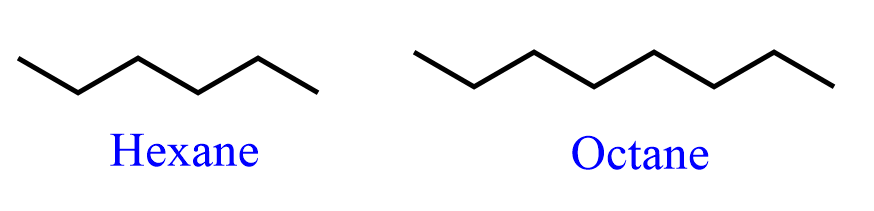
These are called zig-zig structures (bond-line), and we can see the reason by looking at longer hydrocarbons. The hydrogens are most often not shown unless needed for Newman, sawhorse, or stereochemical reasons:
So, what are the sawhorse projections? A sawhorse projection of a molecule is simply a look at it from an angle. This angle is in between the sideview and the front view. The side view gives the zig-zag (bond line) representation, whereas the front view gives a Newman projection.
Very often, it is difficult to understand the conversion from the bond line to Newman projection as the prospective changes too abruptly. For example, this is how the zig-zag and Newman projections of ethane compare:

It is confusing if you are seeing Newman projections for the first time. However, changing the viewing angle not so drastically leads to a sawhorse projection which makes the transition more visual:

It is important to mention that a molecule can have multiple sawhorse and Newman projections because there are drawn based on the direction we are looking from. Therefore, there is no correlation as to a wedge group on the zig-zag is always on the right, or a dash group is on the left, etc. And this is a common confusion pattern when you are just learning the Newman projection.
Think about it like this – Just because the dog’s tail is on the right, doesn’t mean it is always a correct statement. It depends on the direction you are looking at the dog:

The sawhorse and Newman projections are also very helpful when working for larger molecules. For example, let’s draw the Newman and sawhorse projections for the following molecule:
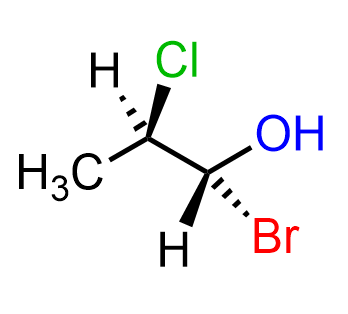
Now, when asked to draw the Newman projection, a direction must be given. Here you can see that Cl is on the top right (facing the right hand), H is on the top-left (left hand) and methyl is pointing straight down. And that is all there is to the first carbon:
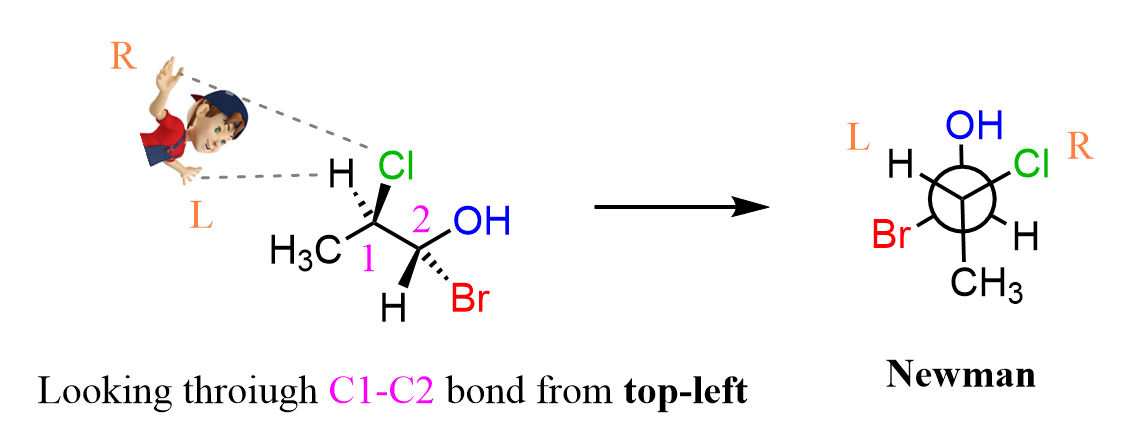
On the second carbon, we have an OH pointing straight up, a Br on the bottom-left, and an H on the bottom-right. The sawhorse projection is in between the bond line and Newman projections:

Sawhorse Projections for Eclipsed and Staggered Conformations
Before talking about eclipsed and staggered conformations, remember that there is a free rotation about single bonds. The simplest molecule to show this would be ethane. If we rotate about the C-C bond, the hydrogens appear at different orientation to one another:
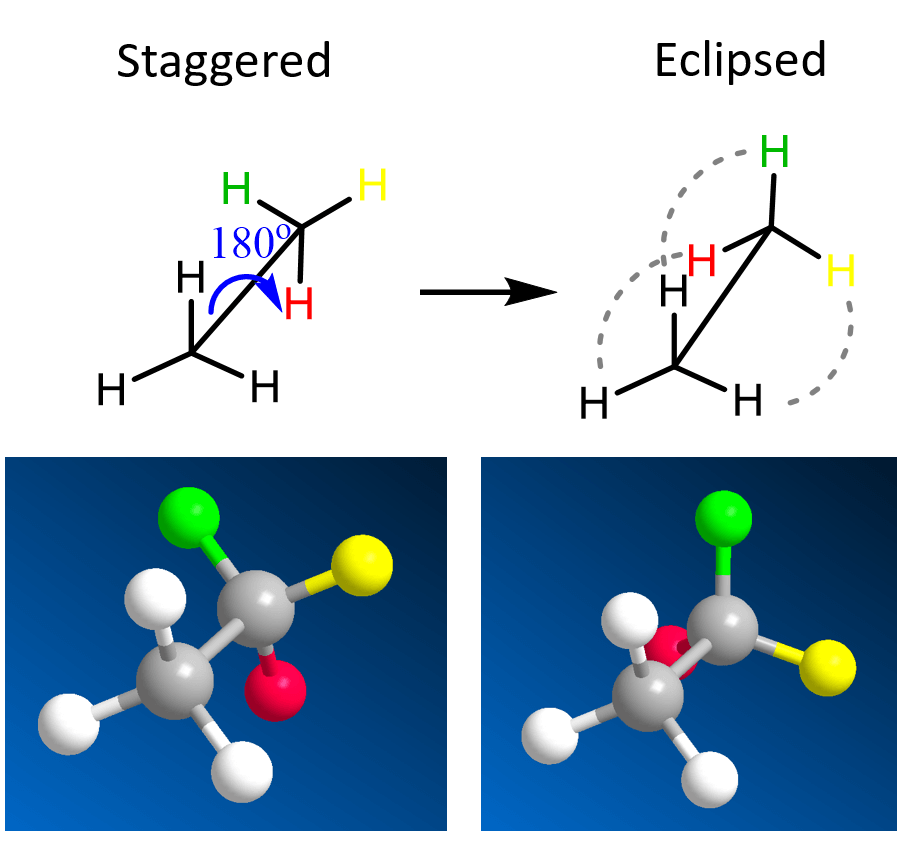
The first conformation is called staggered, and the second is called eclipsed because all the hydrogen atoms are aligned.
We talk more about eclipsed and staggered conformations in the linked article so feel free to check that as well.
Check Also
- Naming Alkanes by IUPAC nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary Secondary and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Practice Problems
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
