From General Chemistry and earlier chapters of Organic Chemistry, we know that C-C single bonds are formed by a linear overlap of sp3 orbitals. As an example, let’s recall how each carbon in ethane provides one sp3 orbital to form the carbon-carbon single bond of the molecule:

One important characteristic of single bonds is the possibility of rotation about them, and as a result of this rotation, a molecule adopts different spatial arrangements of atoms. The different spatial arrangements of the atoms that result from rotation about a single bond are called conformers or conformational isomers.
Depending on whether the hydrogens on the carbon atoms are aligned or not, there are two distinct conformations called eclipsed and staggered. We can show this by wedge and dash representation, sawhorse projection, and most commonly, with Newman projections.
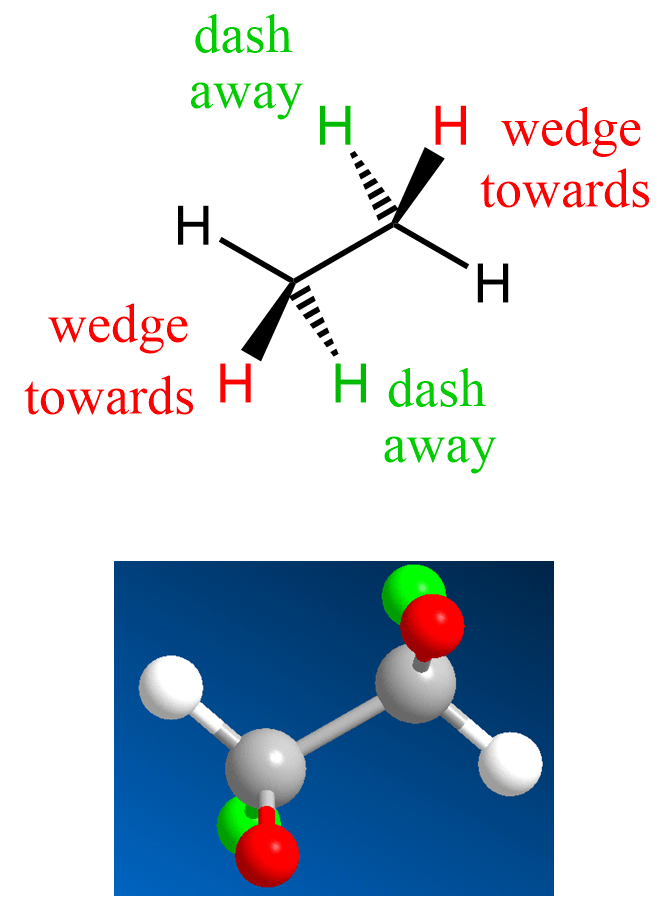
A reminder that in wedge and dash representation, we show the atoms on a tetrahedral carbon (or another atom) being in the plane of the paper as plane lines, those pointing towards us as wedge lines, and the dash lines as used for the atoms pointing away from us. For example, the following is a wedge and dash representation of ethane:
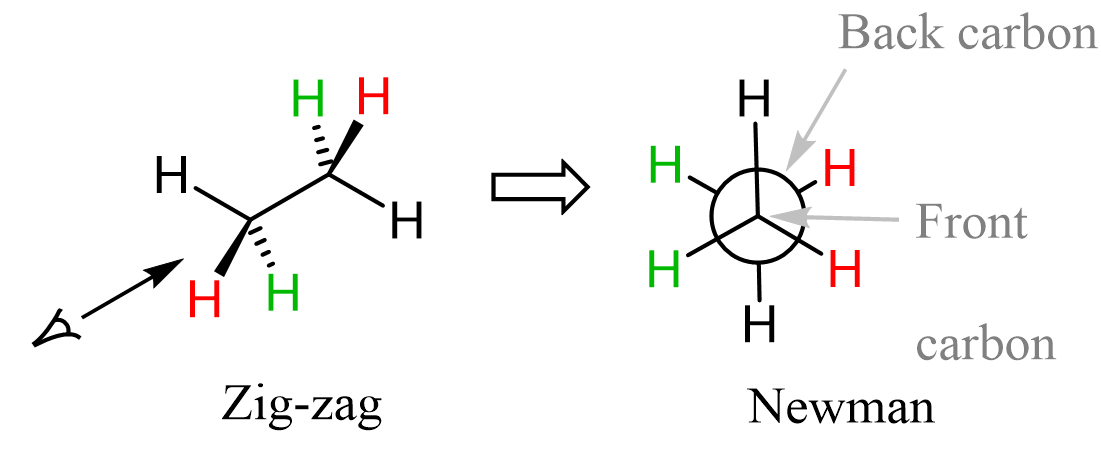
To draw a Newman projection of a molecule, we specify the bond through which we are looking. Therefore, any molecule can have a number of Newman projections for the given conformation.
For the shown conformation for ethane, look from the bottom left, we will get the following Newman projection:
This is the staggered conformation of ethane as all the neighboring hydrogens are at 60o. The continuous rotation about the C-C single bond places these hydrogens at any angle, however, the other important conformation is achieved when the angle is 0o (eclipsed conformation). Coloring the atoms does not matter, we only do it to distinguish them and in the image below, only two neighboring hydrogens are colored green to emphasize their dihedral angle which is the same for all the hydrogens:

Notice that in the first conformation, all the hydrogens are aligned whereas in the second conformation, the angle between the hydrogen atoms is 60o. This angle is called dihedral angle or torsional angle – the angle between atoms on neighboring carbons.
The Stability of Eclipsed and Staggered Conformations
The dihedral angle in the eclipsed and staggered conformations is 0o and 60 o in the respectively. If we imagine the atoms as spheres, it is intuitive to predict that the staggered conformation should be more stable than the eclipsed conformation due to steric effects (the obstruction caused by the size of neighboring groups and atoms).
It is true that staggered conformations are generally more stable than eclipsed conformations unless there are additional factors such as hydrogen bonding which are only possible in an eclipsed conformation. There is no hydrogen bonding in ethane and, as expected, the staggered conformation is more stable.
However, space model calculations have shown that hydrogen atoms are too small to get on each other’s way. The steric effect constitutes only for up to 10% of the destabilizing interactions in the eclipsed conformation of ethane.
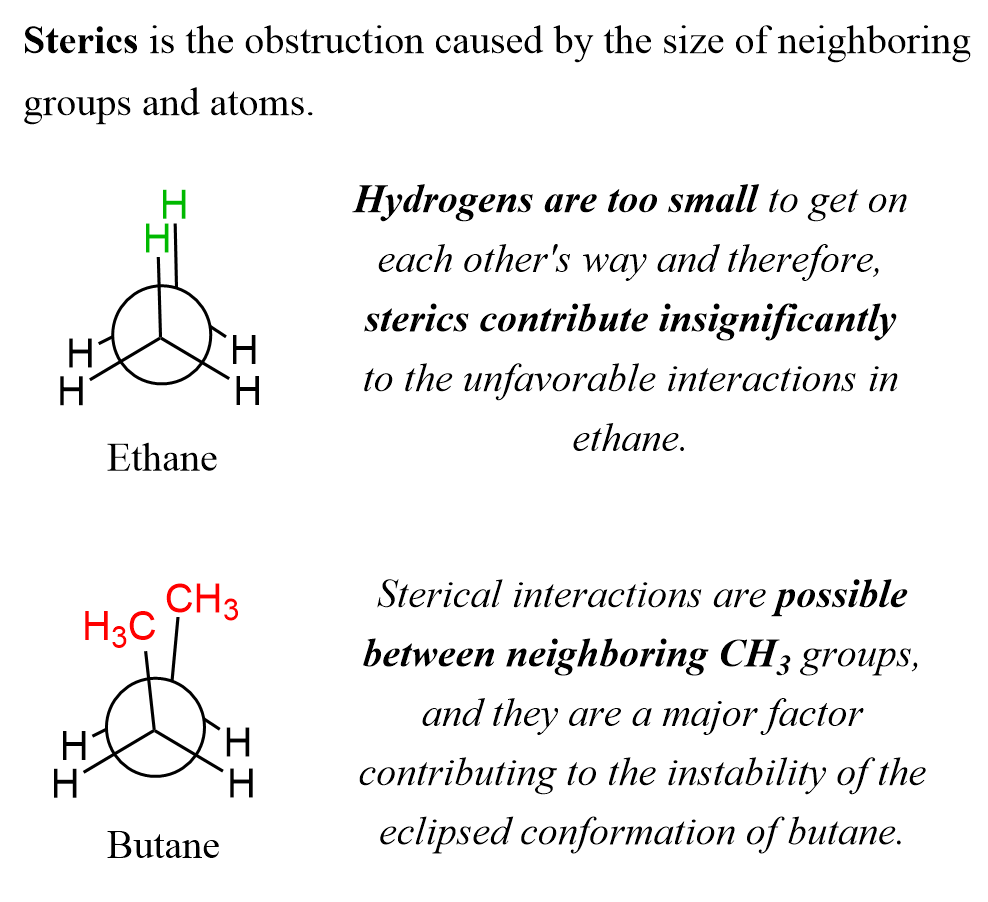
Notice that for larger groups such as methyl, sterics are possible, and these interactions are the major destabilizing factor in the eclipsed conformation of butane.
In ethane, on the other hand, the main destabilizing factor is the electron repulsion between the aligned C-H bonds. Another factor contributing to the energy gap between these two conformations is the stabilization of staggered conformation by the anti-periplanar arrangement of the C-H bonding orbital with the antibonding orbital of the other C-H bond on the neighboring carbon. This is called hyperconjugation:

It might sound confusing and scary, but just remember that, according to molecular orbital theory, each bonding sigma orbital has an antibonding orbital right across to it at 180o. The antibonding orbital is empty and higher in energy, and it is stabilized by the electron-donation of the neighboring C-H bonding orbital.
To summarize the reasons for the greater stability of the staggered conformation, remember that it is stabilized by hyperconjugation, and in addition, the eclipsed conformation is destabilized mainly by the torsional strain which is due to the electron repulsion of adjacent C-H bonds.
Conformational Analysis of Ethane
Conformational analysis is the study of the relative stabilities of different confirmations of a molecule. It is important to mention that ethane and most other molecules where there are no restricted factors such as, for example, a double bond or large groups, exist in an infinite number of conformations. This is because the rotation about all the single bonds continues and happens thousands or millions of times in a second depending on the temperature, media, and the structure of the molecule. We only focus on the distinct/extreme conformations with the largest energy gaps because these are where the molecule spends the most and least amount of time, and thus they define its physical and chemical properties.
For ethane, these are the staggered and eclipsed conformations, and it has been shown that the energy barrier gap between them is 12 kJ/mol (2.9 kcal/mol):
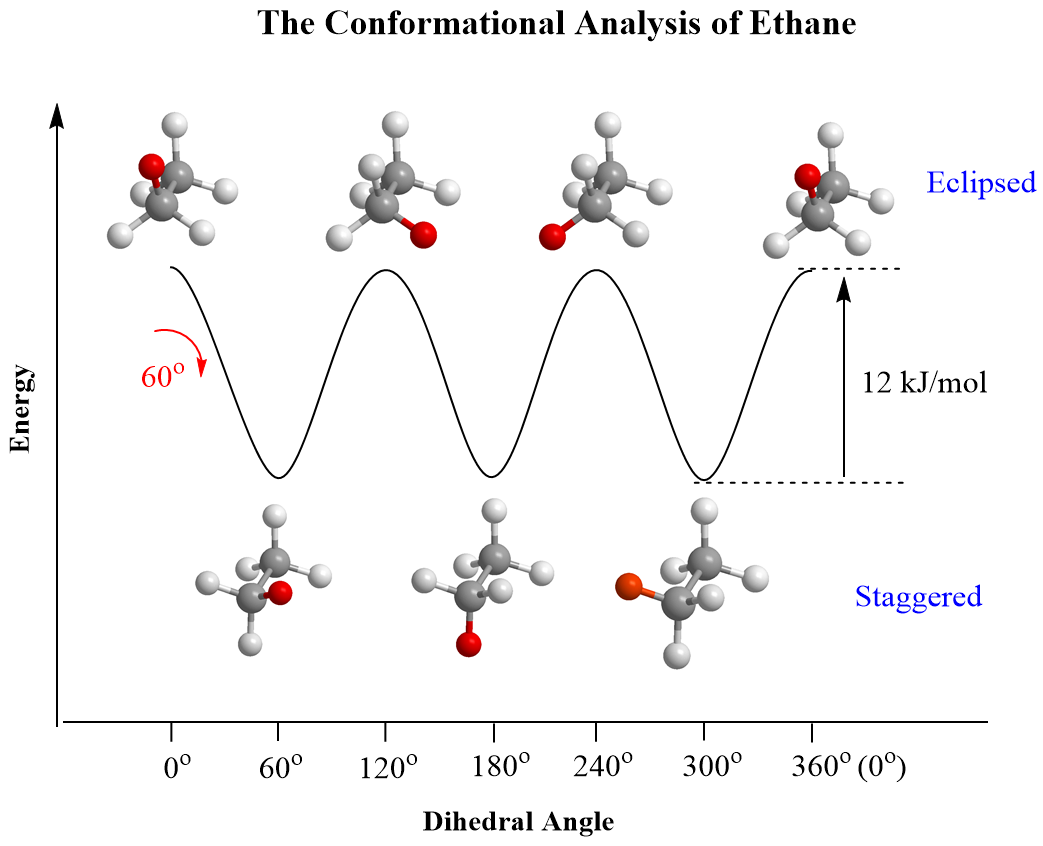
This is the barrier to rotation in ethane, which is defined as the energy difference between the most stable (lowest energy) and the least stable (highest energy) conformations. There is continuous rotation in ethane because 12 kJ/mol is a very low barrier, and at any time, approximately 99% of the ethane molecules are in a staggered conformation.
For comparison, the barrier to rotation about carbon 2 and 3 in butane is 19 kJ/mol because it involves overcoming the unfavorable energy of eclipsing CH3 groups with each other and hydrogen as well as gauche interactions which we will discuss in a separate post dedicated to the conformational analysis of butane.
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Newman Projection of Chair Conformation
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz

Wonderful.i loved the way you explained the whole topic .
Glad to hear it was helpful!