Conformations
Let’s first talk about conformations. These, also referred to as conformers or conformational isomers, are different arrangements of atoms that occur as a result of rotation about single bonds. For example, in the following molecule, we can have a different arrangement of atoms by rotating around the middle σ bond:

Most often, these rotations occur very fast at room temperature that is why the conformations are not considered as different compounds. So, for now, remember that just because the structures look different it does not mean that they represent different compounds:

It becomes a different compound when atoms are connected differently (constitutional isomers), or it is not possible to interconvert between different arrangements of atoms by rotations around single bonds. Good examples are cis and trans isomers, e and z isomers, which are considered diastereomers – a class of stereoisomers. Yes, it gets a bit overwhelming, but let’s deal with conformations and Newman projections for now.
Conformations can be shown by Bond-line (zig-zag), Sawhorse, or Newman projections:

We did a lot of practice on Bond line structures and conversion between Lewis and bond-line structures before: Bond-line, Lewis, and Condensed Structures with Practice Problems
Newman Projections
We said that conformations are different forms of a compound originating through a rotation about a single (sigma) bond. Sometimes, a better perspective of this rotation and the forming conformations is obtained when looking through the sigma bond. And this is what we call a Newman projection.
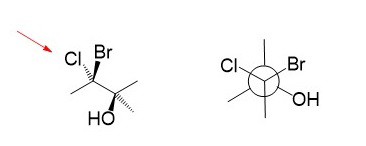
A Newman projection is a representation of the molecule looking through a C-C single bond.
For every Newman projection, you need to specify the bond and the direction you are looking at. For example, for our molecule, we can look through the C1-C2 bond (even though it can be through any bond).
The direction is usually shown with an eye symbol:

So, in this case, if we are looking from the top-left, the carbon in the front is going to be carbon 1, and behind it, we have carbon 2. The corresponding Newman projection would look as follows:

The circle is an imaginary object that is put between the two carbons, so the groups on each carbon are identified clearly in the Newman projection.
This was not so clear, right?
How did the groups appear where they are in the Newman projection?!
Well, let’s replace the simple eye symbol with this fellow. And the reason for this is it is important to see which groups point where i.e. up, down, top-left, top-right, etc.
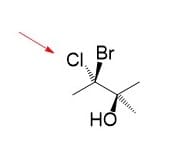
Here you can see that Cl is on the top right (facing the right hand), H is on the top-left (left hand), and methyl is pointing straight down. And that is all there is to the first carbon:

On the second carbon, we have an OH pointing straight up, a Br on the bottom-left, and an H on the bottom-right. Much better than the eye, it should be.
A Molecule Can Have Multiple Newman Projections
As mentioned earlier, for drawing a Newman projection of a molecule, the bond and the viewer’s angle must be given. Depending on the angle, the Newman projections of a given molecule may look completely different. For example, if we were to look through the C1-C2 bond of the previous molecule, from the bottom-left, this is what we’d get:

These two Newman projections represent the same compound, which we can confirm by flipping one of them 180o through an axis:

Notice also that the template pattern for the two Newman Projections is different. In the first one, the front carbon has the “Y” shape, while in the second one, it is an upside-down “Y”. Or we can also call them front-up, back-down, and front-down-back up.

This simply depends on the zig-zag pattern, and if you visualize placing a model with open arms instead of a simple eye, you will make it a lot easier to get the correct pattern.
So, one thing to remember here is that there is no correlation as to a wedge group on the zig-zag is always on the right, or a dash group is on the left, etc. And this is a common confusion pattern when you are just learning the Newman projection.
Think about it like this – Just because the dog’s tail is on the right, doesn’t mean it is always a correct statement. It depends on the direction you are looking at the dog:

The practice is the only way around this and most concepts in organic chemistry. Simply take a paper and draw what you can as much as you can – don’t think too much without drawing anything. If something is wrong, you can fix it, but if you don’t draw anything, you won’t know what it is that you need to work on.
Staggered and Eclipsed Conformations – Dihedral Angle
Let’s take our molecule and do a 180o rotation of the first carbon around the C1-C2 bond:

And now, let’s draw the Newman projection, still looking from the top-left:

What we notice is that all the groups on the back carbon are exactly behind the ones on the front carbon. They are all aligned. This is called the Eclipsed conformation (Lunar eclipse).
In other words, the angle between each eclipsing group is 0o. The angle between these groups is called the Dihedral angle.

Notice that the dihedral angle in all the Newman projections we did before was 60o. It was bisecting the groups on each carbon. All these conformations were staggered conformations – the dihedral angle between all the front and back groups is 60o:

Staggered conformations are more stable than the eclipsed conformations since atoms like space and the closer they come, the more unstable the conformation becomes.
Depending on the dihedral angle between the two larger groups, the staggered conformation can be Anti (180o) or Gauche (60o).

A good example is butane:

Below is the energy diagram of all the staggered and eclipsed conformations of butane. To understand how the transformation from conformation to another occurs, imagine holding the front carbon steady and rotating the back carbon by 60o. Of course, in reality, they both rotate, and we only do this to make it easier to visualize. You could also keep the back carbon steady and rotate the front carbon:

The closer you put the large methyl groups, the more unstable the conformation becomes. Or, which is the same to say, the greater the distance between the large groups, the more stable the conformation is.
Also, the larger the groups, the more unstable the eclipsed and gauche conformations.
Notice that any staggered conformation is more stable than the most stable eclipsed conformation, and gauche conformations are less stable than the anti conformations.
You can read more about the energies associated with different conformations described as Torsional and Steric strain in the next post.







I took Orgo 1 last semester and still get confused with the wedge and dash and left and right in the Newman projection . The dog tail was a good illustration!
Do you have more practice problems?
Most topics have associate practice problems. As far as Newman projections, I am planning on adding more but for now, you can also do the quiz on alkanes and cycloalkanes.
I think that it is a visual exercise that is very easy to understand, and it helps us a lot to understand the subject, I have been working with this type of visualization in my organic chemistry class and it has been really useful to be able to place yourself spacially with the molecule to make correct projections .
so great job, it’s very easy to understand, thanks.
Great to hear this, Yassica. I have been fortunate enough to work in small classes and get the Q&A discussion to the point where I had to position the students and myself in front of the molecule and basically force the visualization of the Newman and Fischer projections for each of them.
Pienso que es un gran ejercicio visual y muy fácil de entender, nos ayuda bastante a entender el tema. He estado trabajando en este tipo de visualización en mi clase de química orgánica 1, y es realmente útil para ubicarnos espacialmente con la molécula y hacer proyecciones correctas.
Gran trabajo, es muy fácil de entender, gracias.
hi
i have a question about diagram of energy comparing between the conformations.
how could i draw this chart with excel or other programs?