Recall, from General Chemistry, that some reactions such as the combustion of methane, ethane, or propane where the molecules were given by their formulas (CH4, C2H6, C3H8).
This is how we work with molecules at the beginning of general chemistry when learning balancing reactions, stoichiometry, limiting reactant, etc. For example, this is how we represent ethanol in its combustion reaction:
C2H5OH + 3O2 → 2CO2 + 3H2O
After this, we get to Lewis structures, and instead of simply giving the formula, we draw out the bonds and add the lone pairs when needed.

Now, one of the first and key differences between general and organic chemistries is that here, we show molecules in 3-D representation. The 3-D structure of the molecule is as important as its formula, and you will see this during your organic chemistry 1 and 2, and even more advanced subjects such as biochemistry and medicinal chemistry.
The simplest organic molecule is methane (CH4) where the carbon is sp3-hybridized and has a tetrahedral geometry. To show this geometry, two of the hydrogens are drawn in the plane of the paper, and the other two are pointing toward and away from the viewer. This is where the wedge and dash notation is used: the atoms pointing towards us (in front of the plane) are shown with a wedge line, and the one pointing away (behind the plane) from us is represented with a dashed line:
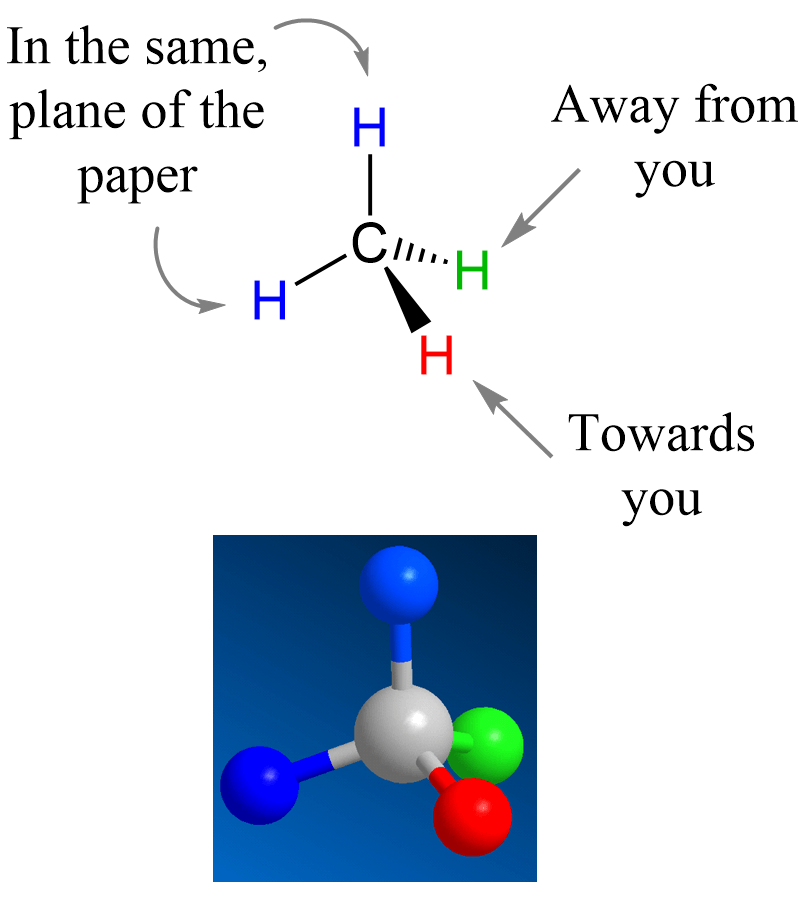
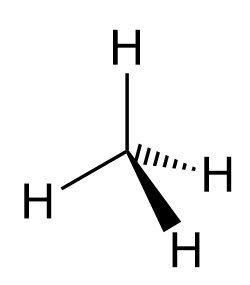
Most often, after the introductory chapters, organic molecules are going to be shown in bond-line representations where the carbon and hydrogen atoms are not shown except when connected to heteroatoms. So, for methane, we can also show it as:
Let’s now look at the wedge and dash notation for the hydrogens in ethane. We have two sp3 carbons connected, and therefore, on each is going to be a wedge and a dash hydrogen:
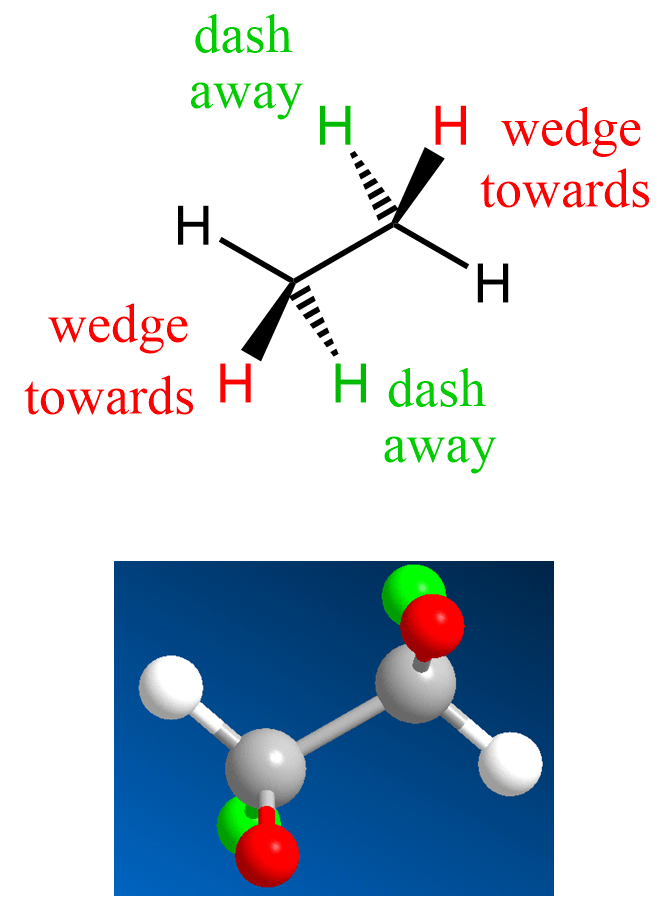
Notice that as drawn, the other two hydrogens are in the same plane as the carbon atoms. Keep in mind, however, that there is a free rotation about single bonds, and therefore, these hydrogens are not fixed in the same plane. It is rather the most convenient way of drawing them.
Here is one important thing you need to remember when adding the hydrogens or other groups on the zig-zag of the main carbon skeleton. When the zig-zag angle is pointing up, you must point the wedge and dash groups up. When the angle is pointing down, you draw the wedge and dash group down as well:

The same principle is applied to larger and more complex molecules: on each sp3 carbon, you add one wedge and one dash hydrogen or any other atom or a group. For example, 2-chlorobutane can have a wedge or a dash chlorine, and these are different compounds, so we need to emphasize that:
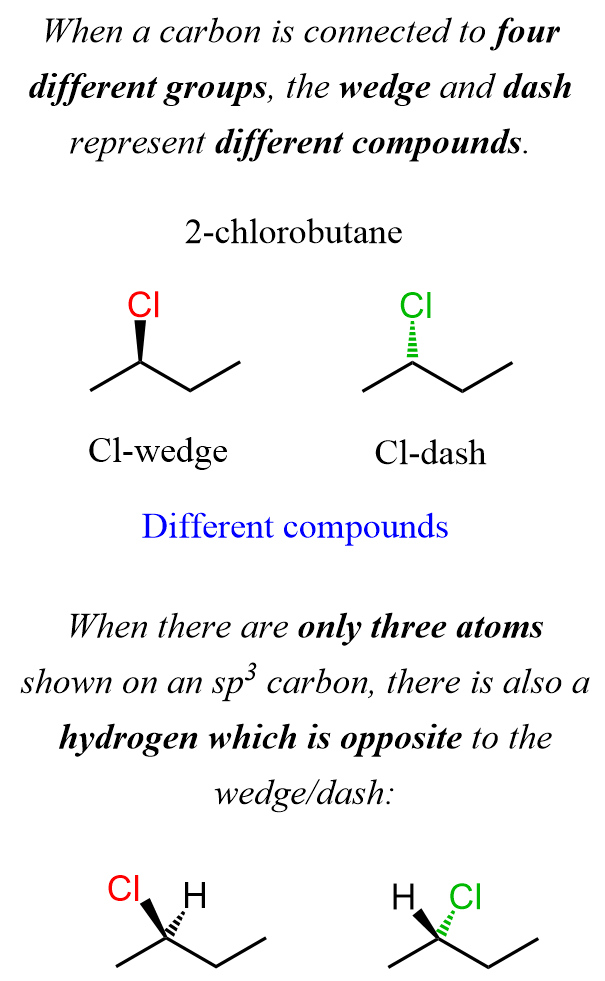
Notice that there are four different atoms connected to the carbon bonded to the chlorine, and this is the reason why having the Cl shown as sedge or a dash makes different compounds. Remember also that there are only three atoms shown on an sp3 carbon, there is also a hydrogen which is opposite to the wedge/dash group.
What is also important to remember is that wedge and dash is not an absolute representation of the special arrangement of atoms because they depend on the direction we are looking at the molecule:

To clear this ambiguity, the R and S notation is used which indicates the absolute configuration of the carbon with four different atoms. This is stereochemistry and you do not have to worry about it yet. The upcoming topics are going to be different representations of molecules such as Newman and Sawhorse, and Fischer when you stat stereochemistry.
Check Also
- Naming Alkanes by IUPAC nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary Secondary and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Practice Problems
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
