Today, we will talk about another type of stereoisomers, which are called diastereomers. This may be overwhelming since at this point you are probably trying to keep track of constitutional isomers, stereoisomers, enantiomers, and not being tricked when the same molecule is drawn differently. So let’s go back a little bit before getting to diastereomers.
In the previous post, we learned about enantiomers, which are a type of stereoisomers that are non-superimposable mirror images.

Remember, stereoisomers are compounds that have the same connectivity but different spatial arrangement of atoms.
For example:

Both pairs here represent enantiomers since the molecules are non-superimposable mirror images. In the first pair, the Br is in position 2, and it is pointing toward you, while in the second molecule, it is pointing away.
Check the post about enantiomers if it is still confusing, as it has more examples that are not restricted to molecules only.
Remember also that constitutional isomers are different from stereoisomers in that they have different connectivity of atoms.
Constitutional (structural) isomers are compounds with the same formula but different connectivity.
Below are a few more examples of constitutional isomers:

Diastereomers
Diastereomers are stereoisomers that are not mirror images.
For example, compare these two molecules:

They have the same connectivity but a different arrangement of the Br groups. The OH groups, however, are identical. So, we have two chiral centers, and only one is changed, while the other one is the same.
Therefore, these molecules are not mirror images (in mirror images, everything is reflected, therefore changed), and this is what we call diastereomers – stereoisomers that are not mirror images of each other.
Let’s put in a little flowchart. Enantiomers and diastereomers are the two main types of stereoisomers. While the stereochemistry of enantiomers is completely changed at all the chiral centers, making them mirror images, diastereomers are not mirror images.

Diastereomers have at least one chiral center where the R, S configuration is the same and one where it is inverted. In other words, in diastereomers, some of the chiral centers are the same, and some are different:
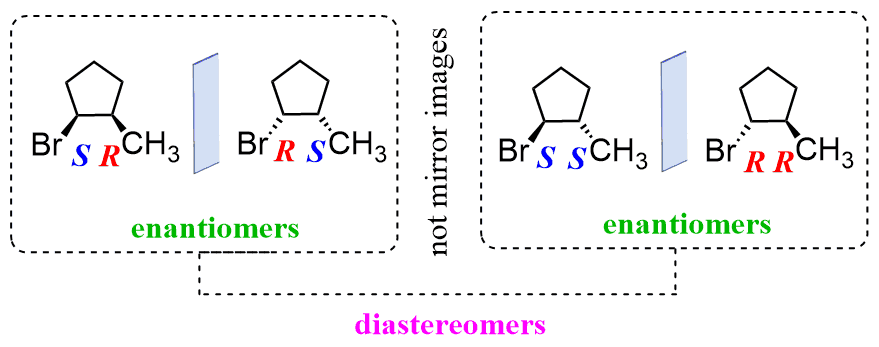
Diastereomers and Cis and Trans Isomers
We said above that diastereomers have at least one chiral center where the R, S configuration is the same, i.e., it is R, R or S, S, and all the others are inverted, or all of them are the same except one that is inverted. In other words, it can have all the chiral centers inverted because that makes two molecules enantiomers, and if they are all the same, then it is the same compound.
This brings a common question of whether diastereomers always have chiral centers, or are they always chiral.
And the answer is no, they do not necessarily have chiral centers. The best example of this is the cis and trans or E and Z isomerism.

Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers. So, Cis and trans isomers are diastereomers.
If the diastereomers are chiral, then the statement about the R and S configuration is correct. However, if they are not, then it is irrelevant to talk about the R and S configuration and chirality in general – they are achiral diastereomers.

Below are some practice problems for mastering the concept of diastereomers and isomerism in general. For some, you will be able to determine the relationship just by visual assessment, some will require determining the R and S configuration, and there are also the ones where you compare different representations, such as Newman vs Fischer, or a bond-line vs sawhorse.
And there is even more to practice! One is this post specifically dedicated to determining enantiomers, diastereomers, and constitutional isomers, and two, you can also take a multiple-choice quiz on stereochemistry and molecular representation, which covers constitutional isomers



Good page
Can someone please explain H and L? For H, I feel that it could be the same and For L, I feel that it could be enantiomers. I don’t think I quite understand the flip part. Thank you in advance.
I am going to make a video on these today.
The video is here. You can also check the stereochemistry quiz which has over 100 solved questions including determining the relationship between two compounds.
Thank you so much!
You are welcome, kimmie. Feel free to ask more questions.