In this post, we will learn how to determine eh absolute configuration of allenes. We are, of course, talking about chiral allenes like, for example, this one:
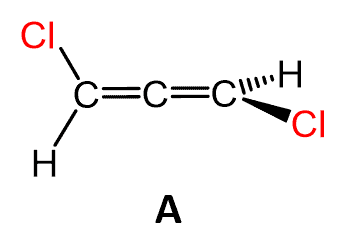
Chirality of Allenes
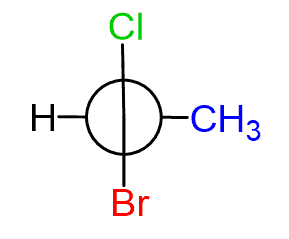
In order for the allene to be chiral, each terminal carbon must be connected to two different atoms/groups. For example, allene (A) is chiral since both terminal carbons are connected to a hydrogen and a chlorine.

Notice that we are talking about two different groups on each terminal carbon. They can still bear the identical group, and the allene is chiral as long as there are no identical groups on one of the terminal carbons:
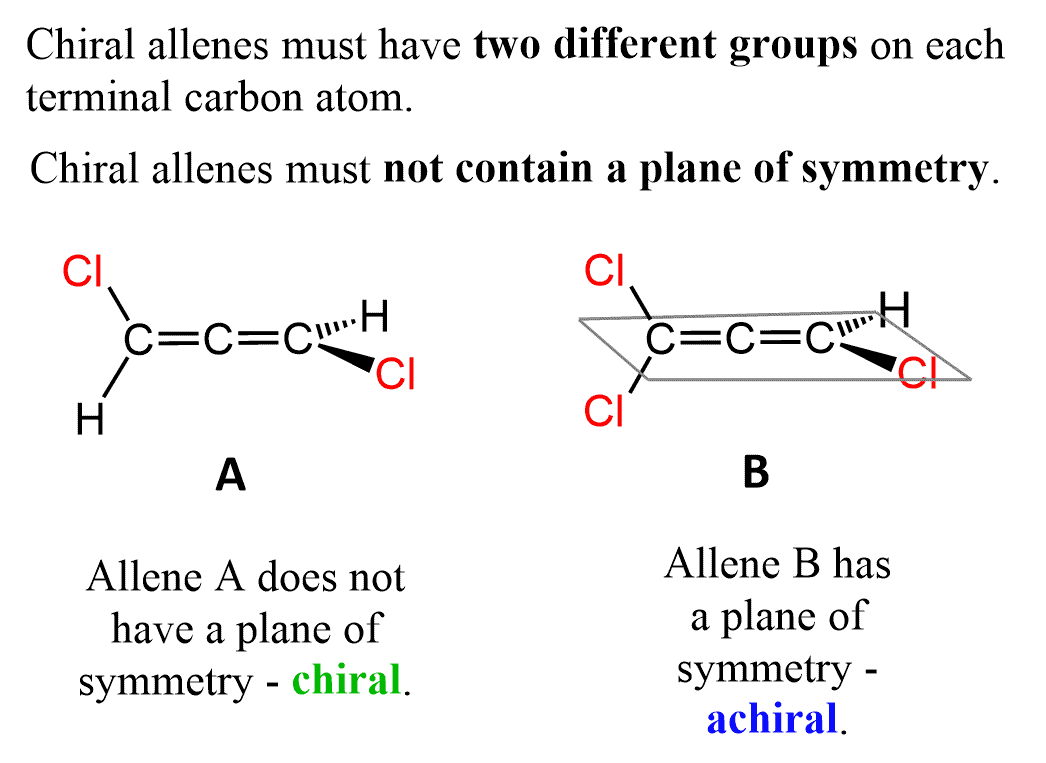
This requirement is based on the principle we learned earlier in the chapter. The molecule is only chiral if it lacks a plane of symmetry. Recall that molecules that contain chirality centers and a plane of symmetry are meso compounds, which are achiral.
The Structure and Geometry of Allenes
Allenes have a linear C=C=C structure where the central carbon is sp-hybridized and the terminal carbons are sp²-hybridized. The central carbon forms σ bonds with the terminals via sp–sp² orbital overlap and has two unhybridized p orbitals arranged perpendicularly, each forming a π bond with the terminal carbons’ p orbitals. This results in two orthogonal π bonds and a unique, twisted geometry where the CH₂ groups lie in perpendicular planes.
As an example, let’s demonstrate how the bonding works on one of the terminal carbons. It is the same for the other, too, and we do this to not overcrowd the image:
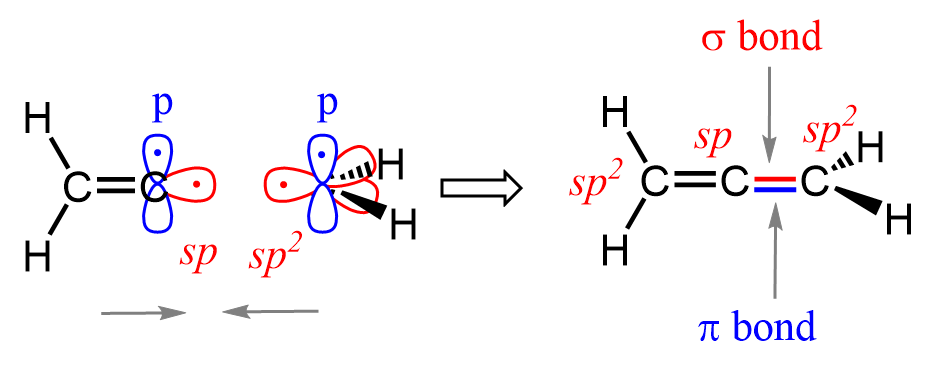
The geometry of allenes is similar to that of CO2, where an sp-hybridized central carbon atom is bonded to two oxygens of sp2 hybridization.
Carbon Atoms in Allenes are Not Tetrahedral – How Can They Be Chiral?
You may be wondering, but wait a minute, how is this even chiral? We have learned through the class that chiral compounds must contain a carbon atom with four different groups.
The type of chirality we talk about in organic chemistry classes is just one type of chirality, known as central chirality, where a single carbon (called a chirality center) is bonded to four different substituents. However, chirality isn’t limited to this! There are other types, like axial chirality, which is exactly what we see in allenes.
There is also what’s called a helical chirality, which originates from a spiral or helical shape in the overall molecular structure. In such cases, the molecule can exist as two non-superimposable mirror images: a right-handed (P or +) helix and a left-handed (M or –) helix.
The most important and well-known example of helical chirality is DNA. Its double helix is inherently chiral and exists predominantly in the right-handed (B-form) in nature.

DNA binding image from PNAS, 2000, 97 (22) 12032-12037
More information in the original article “Allosteric, chiral-selective drug binding to DNA”.
Other examples include helicenes, chiral polymers, and even certain metal complexes.
Absolute Configuration of Chiral Allenes
Some of the principles here follow what we learned about the Cahn-Ingold-Prelog rules in for determining the R and S absolute configuration. More specifically, you will still need to prioritize the atoms/groups and determine the configuration based on the direction of the arrow.
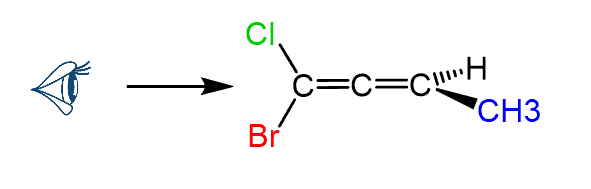
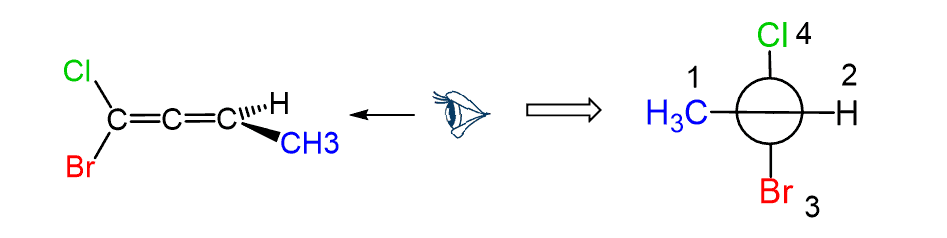
Let’s, as an example, determine the configuration of allene C, where different groups are connected to each carbon:
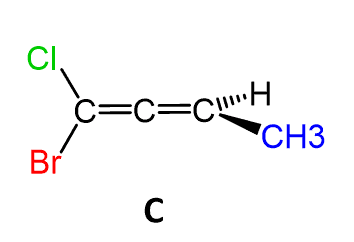
First, pick a direction to look through the C=C=C axis. In this case, we will look from the left side (there is no rule or difference for picking the direction):

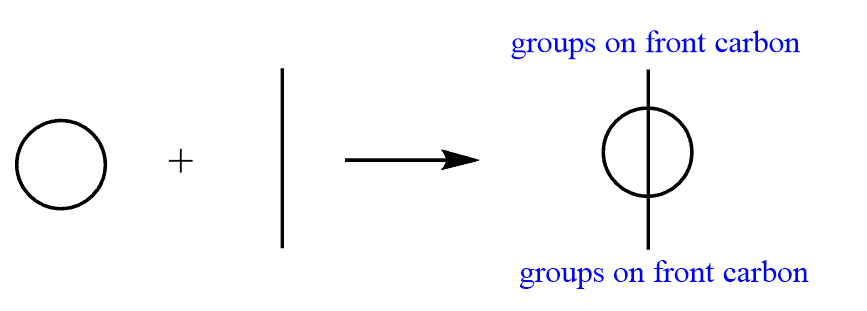
To place the groups on the front and back carbon, draw a circle just like you did when drawing Newman projections, and draw a line over the circle. Again, this line is going to show the groups connected to the front carbon:
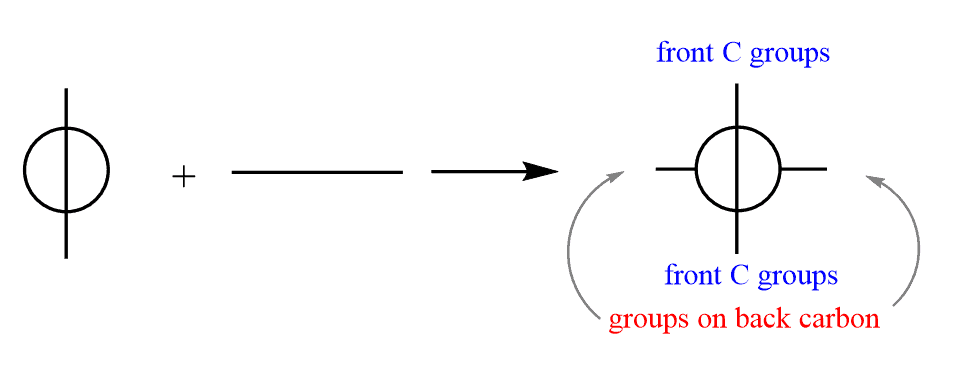
To add the groups on the back carbon, draw a horizontal line behind the circle:
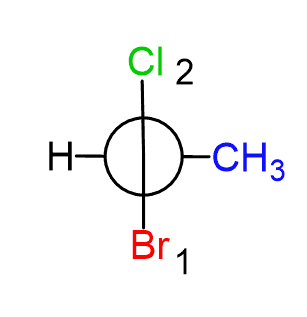
Next, we put the groups. For the front carbon, Cl is on the top and Br on the bottom:
On the back carbon, CH3 and H are on the right and left sides, respectively:

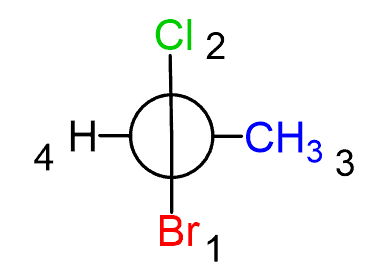
Once we have the structure, groups are prioritized according to the atomic numbers. Importantly, the groups on the front carbon are assigned first, regardless of whether there is a higher priority group on the back carbon.
The groups on the back carbon are assigned as priority 3 and 4.
The arrow goes 1-2-3 – just like in the regular R and S configuration:
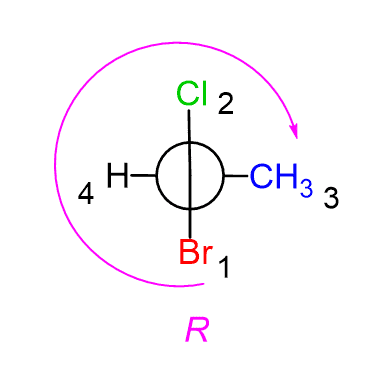
Alternatively, you can simply go from 2 to 3 to make a shorter arrow:
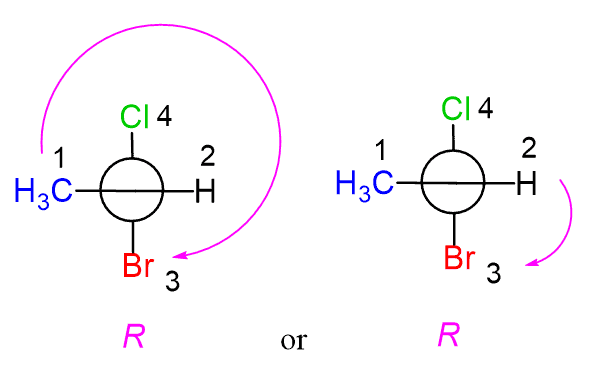
At the end, we can prove that the absolute configuration does not/should not depend on the direction we are looking from. So, this time, let’s look through the C=C=C axis from the right side. This time, the horizontal line is going to be in the front:
The last step is assigning the priorities and determining the configuration based on the direction of the arrow:

Notice again that the groups on the front carbon are assigned first even though, in this case, the ones in the back have higher atomic numbers.
Check Also
- How to Determine the R and S Configuration
- The R and S Configuration Practice Problems
- What is Nonsuperimposable in Organic Chemistry
- Chirality and Enantiomers
- Diastereomers: Introduction and Practice Problems
- Cis and Trans Stereoisomerism in Alkenes
- E and Z Alkene Configuration with Practice Problems
- Enantiomers vs Diastereomers
- Enantiomers, Diastereomers, the Same or Constitutional Isomers with Practice Problems
- Configurational Isomers
- Optical Activity
- Specific Rotation
- Racemic Mixtures
- Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
- Converting Bond-Line, Newman Projection, and Fischer Projections
- Resolution of Enantiomers: Separate Enantiomers by Converting to Diastereomers
- Stereochemistry Practice Problems Quiz

There are 2 steps assigning configuration:
1. Priority rule determines the numbering of groups based on At Nos and
2. Viewing rule, i.e, looking at molecule from the side opposite to the lowest priority group which in a crossed formula for a chiral center (Fischer projection) would require us to place the lowest priority group on the vertical line and then check if it requires to turn clock wise or anti clockwise to establish 1-2-3 sequence that qualifies to designate R or S notation.
Does step two apply to allenes biphenyls and spiranes ? pl enlighten on this.
Unlike Fischer projections, the vertical or horizontal positions in axial chirality do not imply a direction to where the atom is pointing. You can look from both directions and determine the configuration regardless if the lower prioity on any carbon is vartical or horizontal.
Thanks for a prompt reply.
Are chiral allenes always enantiomers?
Like any other chiral molecule, chiral allenes always have their enantiomer – that is, their non-superimposable mirror image.
Thank you for your prompt reply.
My question is: are there any diastereomeric allenes? What I usually see discussed are only the enantiomeric versions. For the same allene molecule, I can identify the Ra and Sa configurations, which are non-superimposable mirror images. However, I don’t see examples of allenes that are diastereomers rather than enantiomers. Also, I tried to rotate the structure, but since rotation around the central double bond is not possible, that doesn’t work—am I understanding correctly?
That is a good question, and you are not mistaken that there is no rotation about the central axis of allenes. I won’t pretend that I have seen this question raised very often in the literature, as it is indeed not discussed much. My take is to think about it this way: a chiral allene has two “chiral centers” – for example, R, R, like in the case discussed earlier. Following the traditional approach, one might say: let’s swap the groups on one of the carbons and get R,S, which should then be the diastereomer of the molecule.
The problem is that by switching one, we actually obtain the enantiomer, and by switching both, we return to the same compound. If this is not immediately clear from the structures, try assigning R and S again – even the one that seems unchanged will flip due to the swap on the other carbon. In other words, the chirality of one “center” in the allene is directly tied to the other.
Notice that switching the groups on different carbons will give constitiounal isomers.
So, the only way of having diastereomeric allenes is if one of the terminal carbons also contains an independent stereogenic center.
Thank you so much
You are most welcome.