In the previous post, we talked about the intermolecular interactions and their correlation with the physical properties of compounds.
The principles of intermolecular interactions are important in estimating the solubility of a given compound. Let’s first define what we understand by solubility. It is the extent to which a compound, called the solute, dissolves in a solvent (usually a liquid in a larger quantity).
Dissolving a compound happens by breaking the interactions between the molecules or ions of the solute and forming clusters via new intermolecular interactions. The process is called solvation.
Therefore, the stronger the interactions between the solute and the solvent the higher the solubility of the solute. And this observation is formulated in the golden rule of solubility: “like dissolves like”.
This means polar compounds dissolve in polar solvents, and nonpolar compounds dissolve in nonpolar solvents.
So, let’s try to answer why like dissolves like.
Just like in the intermolecular interaction of polar compounds, the polar solvent molecules surround with their dipole moments and interact with the solute, thus pulling the compound into the solvent.

The most abundant polar solvent is water, which, aside from having polar bonds, is also capable of making hydrogen bonds. Recall that in general chemistry, we are always using water as a solvent because acids, base,s and salts are all ionic (very polar) compounds and soluble only in water.
Organic liquids, on the other hand, are mostly nonpolar since the hydrocarbon part of them consists of C-H bonds, which are nonpolar and only interact via London dispersion forces. For example, a very common and perhaps the most nonpolar solvent used in organic laboratories would be hexane, as it has no functional groups containing polar bonds. Introducing a group such as an OH to the hydrocarbon chain brings a lot of polarity to the molecule and thus making the solvent more polar. The polar groups are also called hydrophilic, and the nonpolar groups are referred to as hydrophobic.
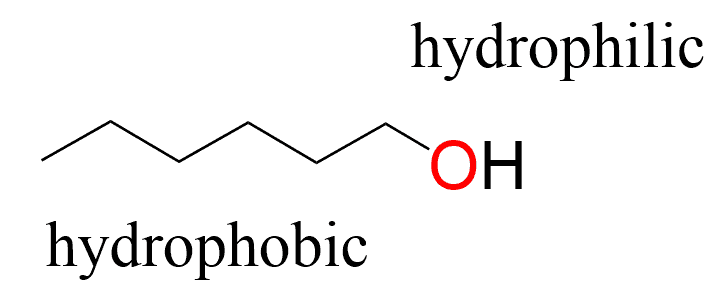
This is demonstrated in the production of soaps/detergents, where the hydrophobic carbon chain surrounds the oily layer forming a micelle, and the hydrophilic part dissolves in wate,r thus washing away the dirt.

The overall polarity of the compound comes from the imbalance of the nonpolar hydrocarbon chain (carbon skeleton) and the presence of polar bonds. For example, methanol, ethanol, and isopropanol are water soluble, while butanol is a lot less soluble than the former. An important example, at the extreme of this series, would be cholesterol, which contains an OH group but is insoluble in water. And the reason for this is the presence of the large carbon skeleton, suppressing the overall polarity coming from the hydroxyl group.

In the examples above, we looked at the molecules from the perspective of being solvents. However, a compound can serve as a solute and a solvent depending on the need. So, methanol is a polar solvent and a polar solute, which is miscible (soluble liquids) in water. It is the most polar organic solvent used for dissolving compounds containing alcohol, amino, and acidic functional groups.
On the other hand, if we mix a nonpolar compound with a polar solvent, the solute and solvent molecules do experience attractive forces, and no solution is formed. The most common example is mixing oil with water, where two distinct layers are formed.

Some common nonpolar solvents are dichloromethane (DCM), tetrachloromethane (CCl4), cyclohexane, benzene, and toluene. These will all be great for dissolving nonpolar molecules, although benzene is carcinogenic and is not used as much as toluene.

Between Polar and Nonpolar Solvents
As we mentioned, the overall polarity of the molecule comes as a result of the imbalance between the nonpolar carbon skeleton and the polar functional group(s). For example, the carbonyl group is polar because of the polarity of the C=O bond. This can be seen by the fact that acetone, having three carbon atoms, is still miscible with water. The C=O group serves as an acceptor of hydrogen bonding with water.

This does not, however, make the molecule as polar as the OH group does since the latter is capable of being both a donor and an acceptor in hydrogen bonding. In addition, the O-H bond is very polar and significantly contributes to the overall polarity of the molecule. Therefore, isopropanol, for example, is more polar than acetone even though structurally they are quite similar.

Another common solvent containing a carbonyl group is ethyl acetate (EA). Its polarity is comparable to that of acetone; however, ethyl acetate is no longer water soluble because there are four carbon atoms in the molecule.

In addition, it is less dense than water, which allows the formation of two layers to be separated, and this makes ethyl acetate a commonly used solvent in extraction experiments.
How Do I Choose a Solvent?
As a rough estimate, remember that water-soluble organic compounds must have an oxygen or nitrogen-containing functional group. In any case, if the molecule has more than 3-4 carbon atoms, its solubility is going to significantly decrease.
The next very polar solvent, which is also an organic compound, is methanol. This will successfully work when water starts failing with the increasing number of carbon atoms. A little less polar would be ethanol and propanol, consequently.
Other polar solvents, which sometimes work when alcohols fail, are dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and acetonitrile.

DMF and DMSO are quite universal as their carbon atoms take the place of any nonpolar part, while the S=O and the amide parts are very efficient in solvating the polar end of the solute. The problem with these two solvents is their high boiling point, which makes it difficult to remove them once the experiment is over.
In the middle, between polar and nonpolar solvents, are ethyl acetate, dichloromethane (methylene chloride), chloroform, diethyl ether, and tetrahydrofuran (THF):
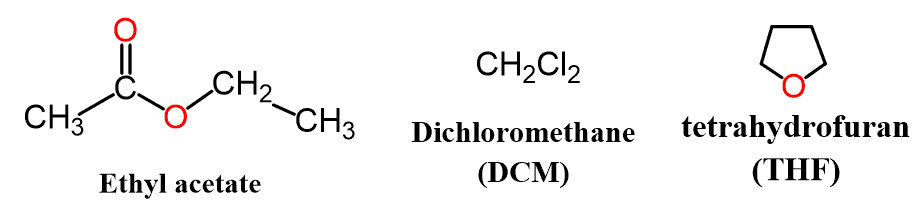
These are great for compounds containing halogens. Remember, alkyl halides are considered polar molecules and are largely used in substitution and elimination reactions.

Although there is no definitive guide to help you determine the perfect solvent in a single shot, when asked to do so, look at the carbon skeleton first. If there are more than 5 carbon atoms, you are likely out of luck picking water or methanol unless there are oxygen or nitrogen-containing functional groups.
To be an “average” molecule in terms of solubility, the solute should have one polar group for roughly 6-7 carbon atoms. In this case, any solvent from acetonitrile to acetone and dichloromethane may be a successful candidate.
If the carbon skeleton largely predominates in the mass of the solute, consider nonpolar solvents such as diethyl ether, toluene, and lastly hexanes and cyclohexane. Some examples of such molecules commonly used in organic laboratories are porphyrins, pyrenes, perylene, and other chromophores. These molecules are extremely hydrophobic unless other functional groups are connected to them.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- Bonding Patterns in Organic Chemistry
- How to Determine the Number of Lone Pairs
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
