In today’s post, we will talk about the IUPAC rules of nomenclature for naming alkanes and alkyl halides.
The first thing you need to do before learning the IUPAC rules for systematic nomenclature is making sure you know the names of the first ten alkanes:

Assuming you have already mastered those, let’s draw a structure and name it simply based on the molecular formula:

The compound has five carbons with no multiple bonds, therefore the formula is C5H12, and based on the common names, we can see that it is pentane.
The complications and need for rules arise when the molecules get branched out. For example, what if we add a methyl (CH3) group to pentane?

This group is considered a substituent, an additional group that is on the “main part” of the molecule called the parent chain.
So, remember, we distinguish two units: the “main part” of the molecule, called the parent chain, and the additional group(s) known as substituents.
There are certain rules for determining the parent chain and the substituent(s) so let’s discuss them one-by-one and name this molecule (let’ name it molecule A) in the course of doing that.
Substituents and Alkyl Groups
The substituent can be a carbon fragment, and these are called alkyl groups, or any other functional group such as a halide, an OH, a nitro group, etc.

Alkyl groups are formed by removing one hydrogen from the corresponding alkane and are named based on this alkane by simply changing the ending from –ane to -yl.

Sec-, tert- and iso- prefixes
Just like constitutional isomers, it is possible to have different alkyl groups with the same chemical formula.
For example, aside from the propyl group, there is also isopropyl. The difference is that in the isopropyl, a hydrogen, connected to a secondary carbon atom, is removed, and it is this secondary carbon that is connected to the parent chain.

Likewise, the butyl group can also be primary, secondary, and tertiary. It will be very helpful to memorize all these groups and below is a general scheme to visualize how the names of these alkyl groups are derived:

You can also read this post about primary, secondary, and tertiary carbon atoms.
Identifying the Parent Chain
The parent chain is determined based on the longest continuous carbon chain that is present in the molecule. As an example, let’s consider molecule A mentioned earlier:

If we start numbering the carbon atoms from the methyl substituent, we can only have a continuous chain of four carbons. However, starting from any end allows making a five-carbon chain, which is preferred since it makes a longer parent chain.

Therefore, the parent chain is pentane, and the substituent is a methyl group.
If you run into a situation where there are two chains of equal length, then choose the one with the greater number of substituents:

When a ring is present, the parent chain is determined based on the number of carbons. If the ring has more carbons than the chain, then it is the parent chain:

Notice that the carbons in the ring belong to the ring only. I.e., you cannot count the carbon twice or include it in the carbon chain.
If the ring and the chain have an equal number of carbon atoms, the ring gets priority and is considered the parent chain.
Putting the parent chain and substituents together
When naming a compound, the alkyl groups are listed first, followed by the parent chain. No sign or space is needed to separate two words.

However, notice also that a number to specify the position of the alkyl groups is included in the final name. And this an important piece of information.
To illustrate this, let’s look at this example.
The following two compounds are both methylpentanes, but they are clearly not identical:

And, in order to distinguish them, we need to specify the location of the methyl group. For this, the parent chain is numbered, and the rule here is to always do it such that the alkyl group gets the lowest possible number:

Starting from the left or the right side of the parent chain, we get two names, and out of these, 2-methylpentane is better than 4-methylpentane. Therefore, 2-methylpentane is the correct IUPAC name of this compound.
Notice that for the second compound, it does not matter where we start the numbering, since it is a symmetric molecule, and either way, the methyl group gets number 3:

A few additional details to point out when writing the name of a compound:
1) Numbers and words are separated by a dashed line
2) Words are not separated by any sign or a space
Parent chain with two substituents
Now, let’s add another methyl group next to the first one:

Again, you have two options for numbering the parent chain. One gives the 3,4 and the other one 2,3 locants for the two methyl groups, and 2,3 clearly beats 3,4.

Therefore, the final name of our compound is going to be 2,3-dimethylpentane.
Notice that numbers are separated by commas and because there are two methyl groups, we need to use the prefix “di” before the name of the alkyl groups.
In general, if two or more identical substituents are present, the corresponding prefixes are used to indicate their number:
Two – di
Three – tri
Four – tetra
Five – penta
Six – hexa
Seven – hepta
Eight – octa
Let’s also consider the other option of having two methyl groups on pentane:

Notice that, in this case, regardless of where we start the numbering, the first methyl gets locant 2, and the second one gets 4. Therefore, it is 2,4-dimethylpentane.

Now, the question comes – what if there is a third substituent and it does matter where to start numbering?
To answer this, let’s consider heptane with three methyl groups:

Starting from left or right makes no difference as far as having the location of the first substituent. Either way, it is 2. However, if you start from the left, you are getting 2,3,6-trimethylheptane, while starting from the right, gives 2,5,6-trimethylheptane.

And because 2,3,6 is better than 2,5,6, the correct name of this molecule is 2,3,6-trimethylheptane.
To summarize this observation, when there is a tie for the location of the first substituent, compare the second one, then the third, till you find a tiebrea,k if there is one.
If numbering the alkyl groups does not break the tie, then the substituent with the alphabetical priority gets the lower locant:
For example, having a bromine and chlorine on both ends of hexane brings up the need of prioritizing the substituents based on their alphabetical order.

In this case, 1-bromo-6-chlorohexane beats 6-bromo-1-chlorohexane:
If none of the rules discussed above give a tiebreak, then it is a symmetrical molecule, and it does not matter where you start numbering the parent chain, as long as you do find the correct parent chain.
Alphabetical order in IUPAC naming
So far, we have considered having identical alkyl groups. Now, suppose we need to name the following compound:

Step 1. Find the parent chain. The longest possible chain here consists of nine carbons, so the parent chain is nonane.

Step 2. Find the substituents. In this case, we have a methyl and an ethyl group.

Step 3. Number the parent chain, giving the lowest possible numbers to the substituents:

Out of the two options, 2-methyl is better than 4-ethyl.
Step 4. Put the parent chain and substituents together by placing the substituents in alphabetical order!

This means that even though the methyl group is at position 2, the ethyl group with the locant 6 is still placed before it:
The alphabetical priority of prefixes
None of the prefixes such as di, tri, tetra, sec-, tert- are considered for alphabetical priority except the -iso.
For example:

Still looking forward to finding out why -iso is privileged…
Naming complex substituents
Sometimes, we run out of the common names for the substituents, such as sec-butyl, tert-butyl, iso-butyl, but we still need to name a substituent that is larger than usual.
For example, in the following molecule, it is easy to spot the ethyl group, but naming the second substituents needs to follow certain rules.
The good news is that these rules are no different than what we use when naming a compound. Essentially, you need to look at the complex substituent as a separate molecule and find its “parent chain” and the alkyl groups on it.
To do this, start numbering from the carbon directly connected to the actual parent chain of the molecule and list the alkyl groups alphabetically:

Notice that at the end, the quasi parent chain gets the -yl suffix since it is still a substituent, and the actual parent chain is placed at the end.
This systematic approach for naming alkyl groups can also be applied to the ones with common names, and you will likely need to know both options.
In the following practice problems, we will go over naming alkanes using the IUPAC nomenclature rules, which include finding the parent chain, numbering it to have the substituents in the correct positions, and finally putting all of this together to name the compound.
The next exercise will teach you to draw the structure based on the IUPAC name.
IUPAC Moments
IUPAC now says that…

Quoting the IUPAC 2013 Blue Book:
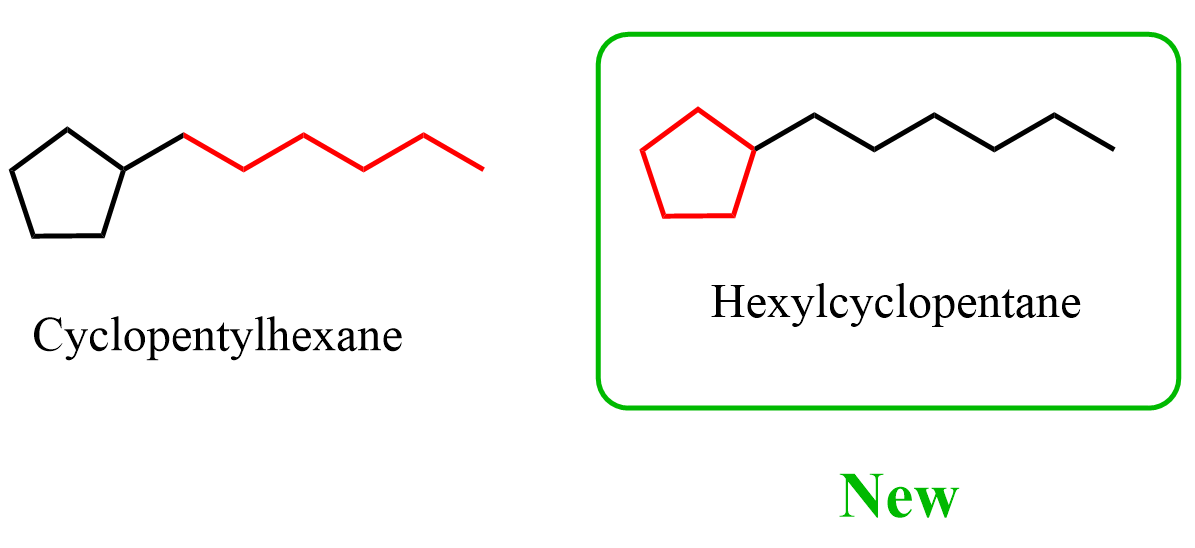
P-44.1.2.2 – Rings Systems composed of rings and chains (exclusive of linear phanes). Two methods are recognized to name systems composed of rings and chains (exclusive of linear phanes). (1) Within the same class, a ring or ring system has seniority over a chain. When a ring and a chain contain the same senior element, the ring is chosen as the parent. Rings and chains are chosen regardless of their degree of hydrogenation. As a consequence, this approach prefers the choice of a ring over a chain in systems composed of cyclic and acyclic hydrocarbons.
So, apparently, hexylcyclopentane is the preferred IUPAC name (PIN) for what we called cyclopentylhexane:
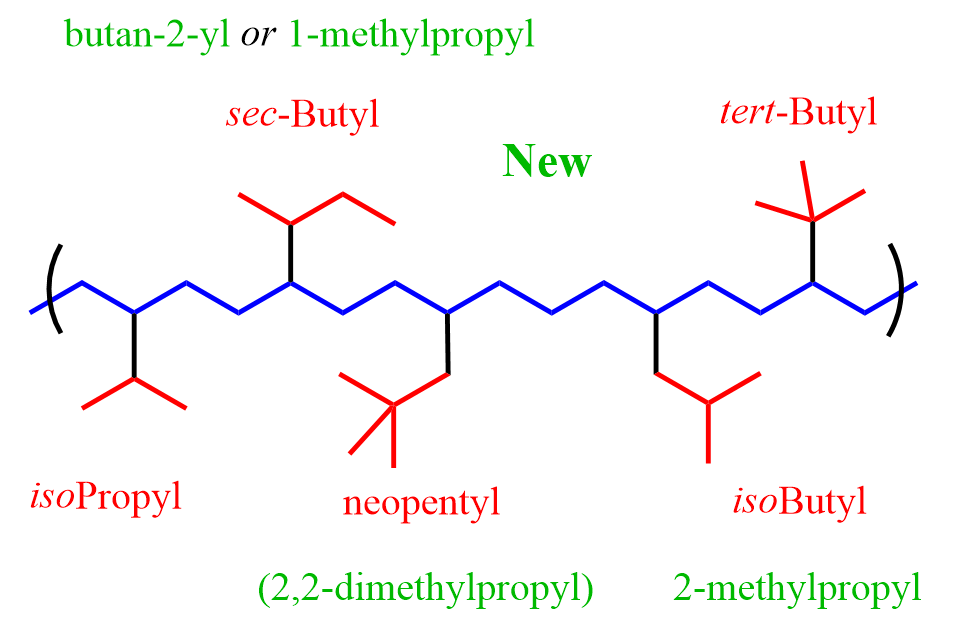
P-57.1.4 – Substitutents Retained prefixes no longer recommended
The retained names phenethyl (2-phenylethyl) for C6H5-CH2-CH2–; benzhydryl (diphenylmethyl), for (C6H5)2CH–; isobutyl (2-methylpropyl) for (CH3)2CH-CH2–; sec-butyl (butan-2-yl, 1-methylpropyl) for CH3-CH2-CH(CH3)–; isopentyl (3-methylbutyl) for (CH3)2CH-CH2-CH2–; tert-pentyl (2-methylbutan-2-yl, 1,1-dimethylpropyl) for CH3-CH2-C(CH3)2–; and neopentyl (2,2-dimethylpropyl) for (CH3)3C-CH2–; are no longer recommended; the first name in parentheses is the preferred prefix name.
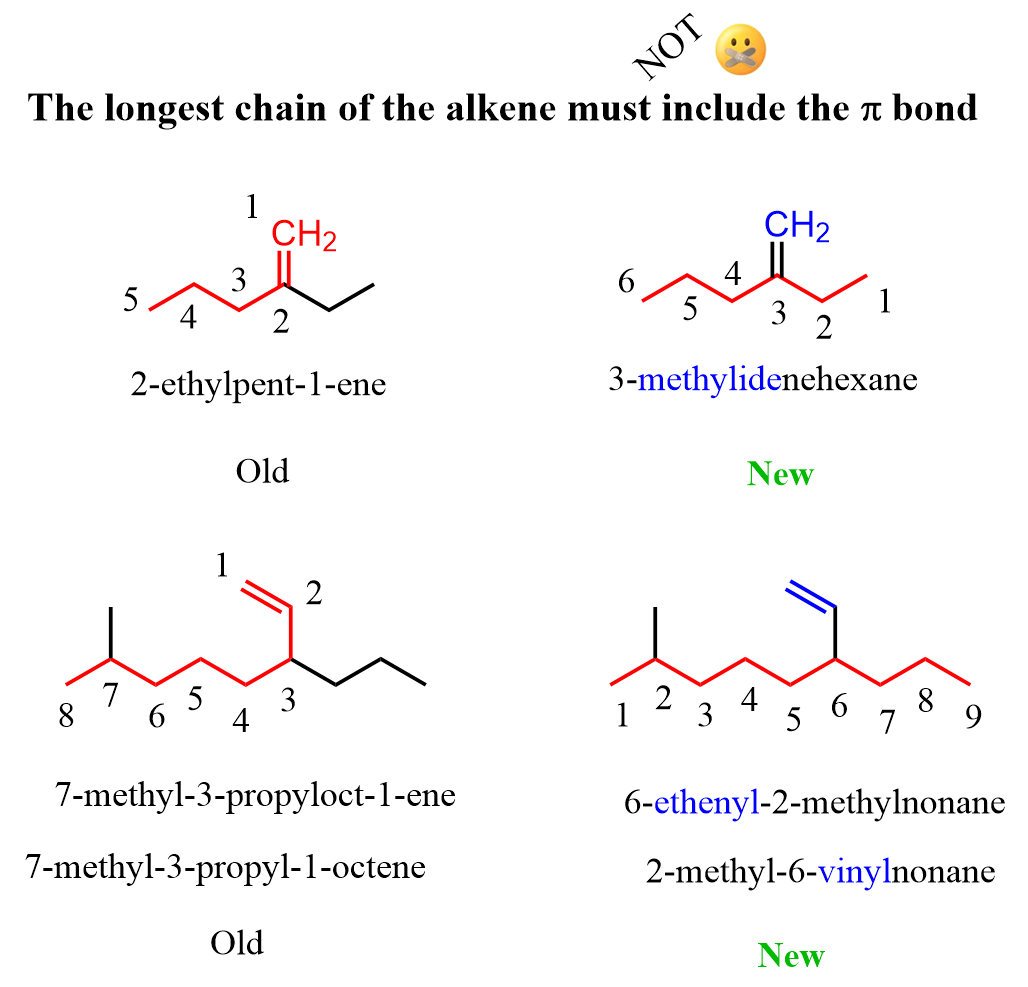
P-44.3 – Unsaturation The parent chain of an alkene must include the double bond. This rule has been modified from previous rules; seniority is now given to the length of the chain rather than to unsaturation.
For example, based on the new rules, this molecule is named 3-mehylidenehexane rather than 2-ethylpent-1-ene:
The last point is related to the nomenclature of alkenes, which we discuss here.
So, which rules do we follow when naming certain alkanes, cycloalkeanes, and alkenes? Honesty, I think this is not the most important part of learning and understanding organic chemistry… In most of the examples and practice problems at Chemistry Steps, we follow the earlier rules as this is what most textbooks, at least in the US, do. Ultimately, it is up to your instructor how they want to address these, so follow the rules given in the class or check with them for clarification if needed.




Can you please tell me the proper name of this compound:
(CH3 )3 C-CH2-CH(Cl)-(CH2)3-C(CH3)2-CH(NH2)-C(C2H5)(CH3)2
You need to first convert this into a bond-line structure to find the parent chain and substituents.
9-amine-4-chloro-2,2,8,8,10,10-hexamethyl n-dodecane
4-amine-9-chloro-3,3,5,5,11,11-hexadodecane
I guess
Sorry’one mistake its 4-amine-9-chloro-3,3,5,5,11,11-hexamehtyldodecane
This is the correct 👆
What is the bond line structure of the above?