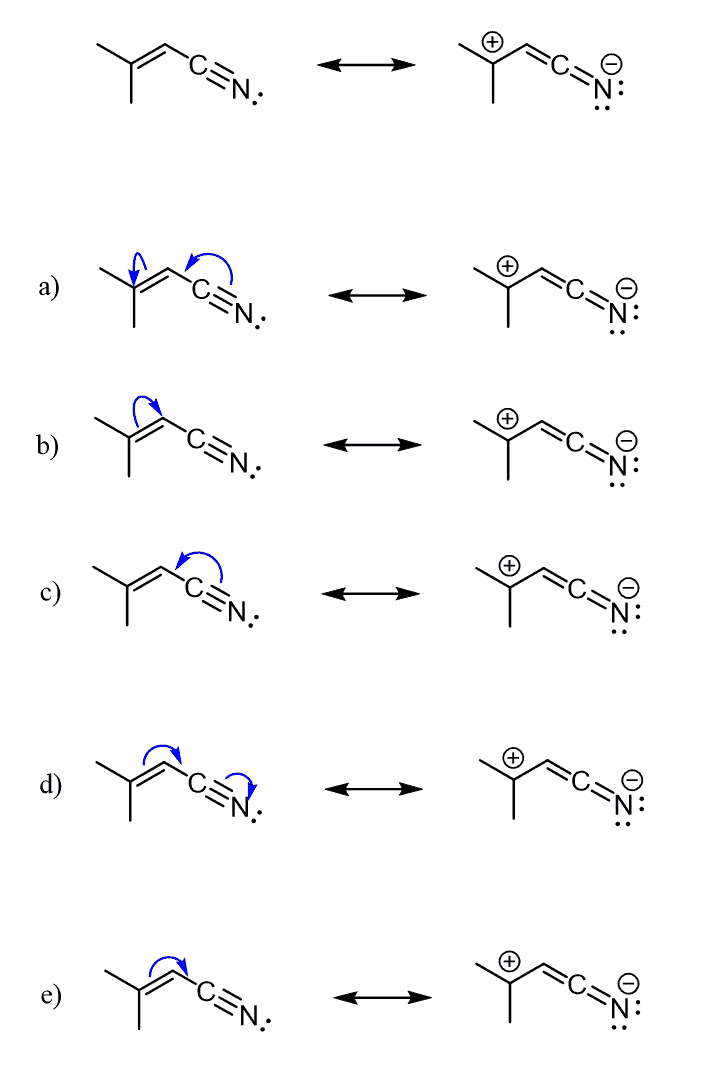
Have resonance structures been a major topic in your class recently?
It may seem that there are too many rules to memorize about resonance structures, but there are a few important things you need to realize before getting into the details of this topic.
First of all, do not memorize any of these!
I guarantee you, there is a chance if you ask your professor in the middle of summer, when they are not teaching an organic chemistry course, they won’t remember most, probably, any of these rules. They won’t tell you anything wrong, though, that is for sure, and that is because they understand what resonance structures are.
So, Rule 1 – Know What Resonance Structures Are
Do you?
Yes, resonance structures are different Lewis structures of the same molecule or ion. The keyword here is “the same,” which means whatever resonance form you draw, keep in mind that it cannot represent a different molecule than the original.
For example, the following two are resonance forms of dimethyl formamide (DMF). They both represent the same compound, DMF:
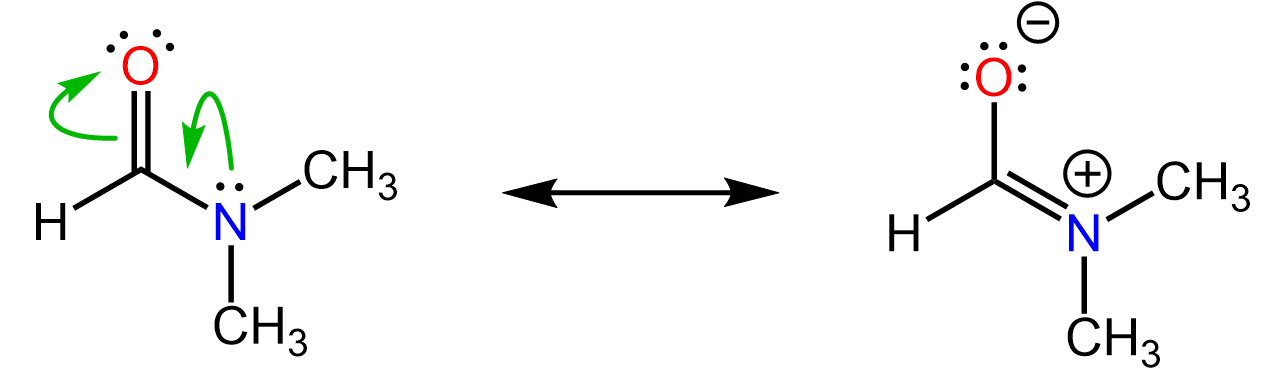
What is different, though, is the distribution of some electrons in the molecule. In the first representation, there is a pair of electrons on the nitrogen, while in the second one, those electrons have moved between the nitrogen and the carbon, making a new C=N π bond.
Notice also that the resonance structures are separated by double-headed arrows. Do not confuse this with the double arrows used to show reversible reactions and chemical equilibrium.
Here are some more examples of resonance structures where you can see that, despite having different electron and charge distributions, all the atoms have the same connectivity. Thus, the structures represent the same species.

This brings us to the second rule of resonance structures. Actually, I think it will be the first rule since we all know that talking about something assumes knowing what it is in the first place. Check this post on resonance structures if you need to learn what they are.
Rule 1: Octet Rule and Resonance Structures
Let’s first emphasize what we mean by the Octet Rule. In organic chemistry, you need to read it as “Do NOT exceed the octet of carbon, nitrogen, oxygen, and halogens.” In other words, you can draw a carbon with three bonds, but never more than four. Of course, you need to have the correct combination of lone pairs and a charge when an atom gets off its standard bonding patterns, so go over this table to review the bonding, lone pair, and formal charge patterns of important atoms in organic chemistry:

Let’s now consider a few examples of correlating the octet rule with resonance structures. Notice that having atoms with nonstandard bonding patterns is ok, as long as you keep track of the number of electrons and corresponding formal charges. Once again, remember to not exceed the octet on, first of all, carbon, second row elements, O, N, F, and other halogens. Unlike in general chemistry, halogens only have one bond in organic chemistry.
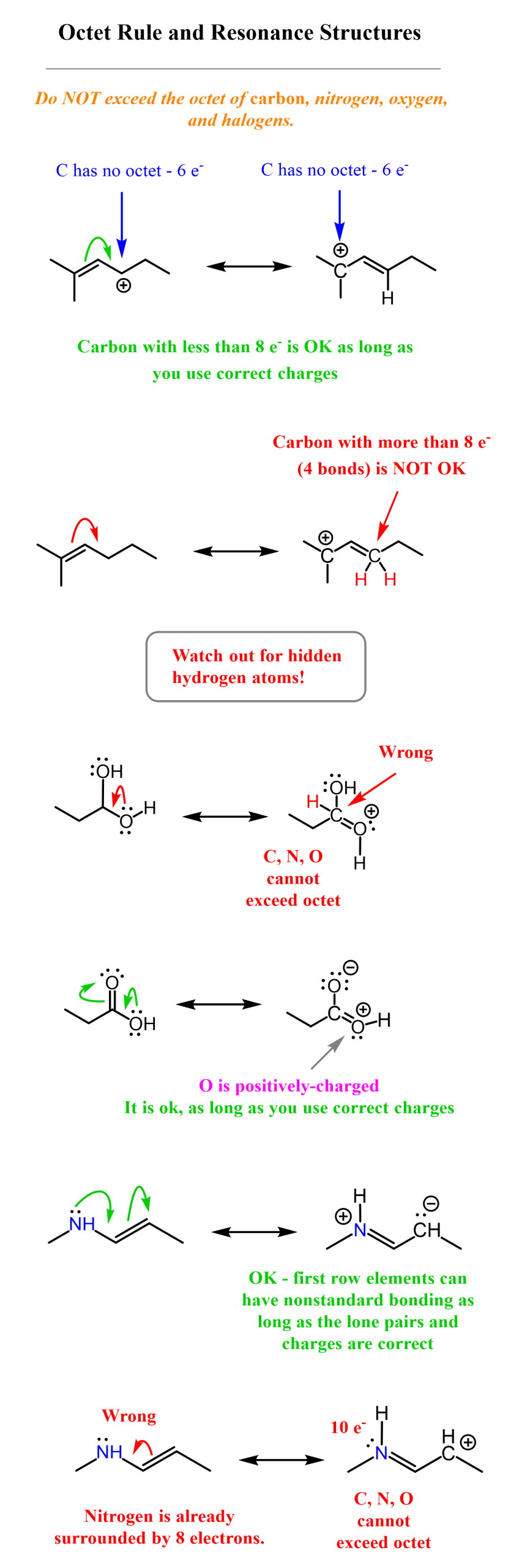
Pay special attention to hidden hydrogens in bond-line structures! This is perhaps the most common reason for making mistakes in arrow pushing for resonance structures. I have added the relevant hydrogens to the structure on the right to emphasize why the structure is correct or incorrect.
Let’s add a few more examples illustrating two important rules of drawing resonance structures: not exceeding the octet and not breaking single bonds.

Rule 2: Do NOT Break Single Bonds
This is what we discussed at the beginning of this post. Breaking a single bond(s) means changing into another molecule/ion, which contradicts the definition of resonance structures – they are different Lewis structures of the same molecule.
For example, to draw a resonance structure for the following molecule, we cannot start the curved arrow from the middle of the C–O bond, because that implies breaking the bond.

Instead, we could use the lone pair on the oxygen to make a pi (π) bond with the carbon by also moving the electrons of the C=C π bond. The second point is important because otherwise, the middle carbon would be surrounded by 10 electrons, thus exceeding the octet.

So, what can we conclude about the types of bonds and resonance structures?
When drawing resonance structures, never make or break single (σ) bonds. On the contrary, they always involve a π bond, either making a bond or breaking one. For example, in the following reaoance transformation, the lone pair on the nitrogen did become a double bond (N=C), which forced the electrons of the pi bond between the two carbons to move to the right one. As a result, there is now a lone pair on that carbon, and it has a formal charge of -1.

To summarize the role of pi and sigma (the lack thereof) bonds, remember, whenever asked to draw a resonance structure(s), look for a π bond. You can’t have resonance structures without having a π bond involved.

Rule 3: Do Not Start Arrows from Single Bonds or Positive Charges
Curved arrows show the movement of electrons, and that is true whether we are using them to show reaction mechanisms or resonance structures. The big difference between the two is that, in chemical reactions, the movement of electrons results in the making and breaking of covalent bonds, including sigma (single) bonds. In contrast, in resonance structures, the connectivity of atoms stays the same. This means that resonance structures represent the same species, just with different electron distributions.
For example, the first mechanism represents a chemical reaction: the movement of electrons from the cyanide ion (⁻CN) to the carbon bearing the bromine atom (C–Br) indicates the formation of a new C–C single bond. The second arrow indicates the breaking of the C–Br bond.

On the contrary, the movement of a lone pair from the oxygen to the carbon shows the formation of a new O=C π bond. This is resonance, not a reaction.
The point of showing a chemical reaction and resonance structure next to each other was to emphasize that curved arrows show the movement of electrons; therefore, you can never start an arrow from a positive charge.
In general, you can either start a curved arrow from a pair of electrons (negative charge is also acceptable) or from the middle of a bond. However, this is important!! In resonance structures, you can only mess around with pi bonds (sorry, I had to say that), therefore, you can only use curved arrows from the middle of a pi bond.

Charge and Number of Electrons in Resonance Structures
There are two more implications of the rules for drawing resonance structures. I would not classify them as rules, since it is impractical to draw resonance structures by focusing on following them:
- The total number of electrons must stay the same
- The overall charge must remain constant
We do not need to count all the electrons of the molecule to check if it stays constant in different resonance structures. We can focus on the part where the electron redistribution occurs. For example, the following lactam (cyclic amide) has two major resonance structures, so let’s count the number of electrons and charge distribution:
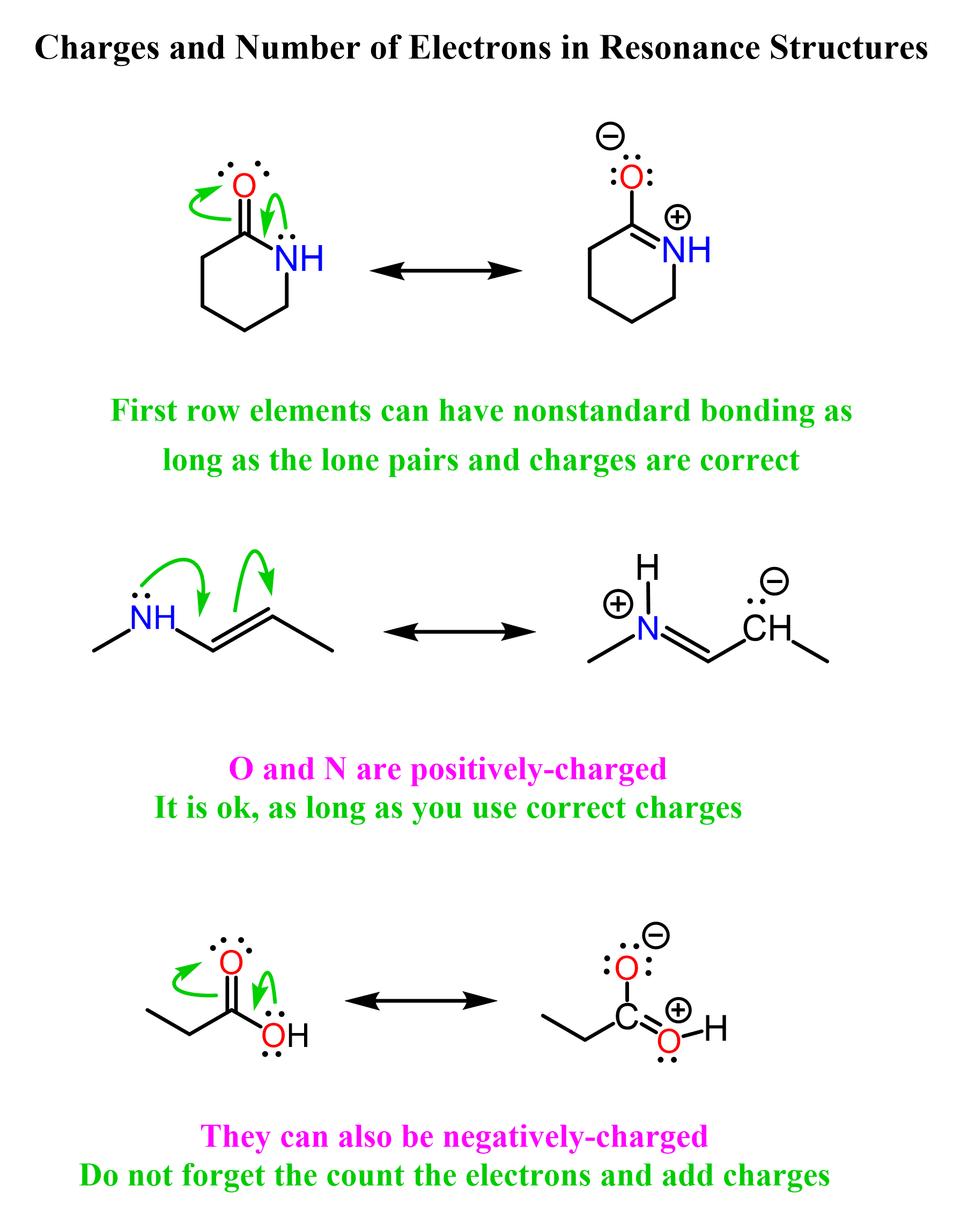
In the first resonance form, there are two lone pairs on the oxygen, 4 electrons making the sigma and pi bonds between the carbon and oxygen, two making the C–N single bond, and a lone pair on the nitrogen. That is 12 electrons in total. In the second resonance structure, oxygen has 6 electrons from the three lone pairs; there are two electrons making the C–O single bond, and four electrons making the C=N double bond. As expected, that also makes 12 electrons.
Notice also that the charge balance is also equal. The first resonance form is neutral, and the second one is technically also neutral as the positive and negative charges balance out. We say technically because having an equal number of opposite charges does not make the molecule neutral as such. For example, common organic species like alkoxides (R–O⁻) have counterions such as Na⁺ and K⁺, but that does not make them neutral.
You see, the discussion keeps evolving, but all of this is just an implication of the fact that resonance structures represent the same molecule. If they are the same, then they cannot have different connectivity, thus a different number of atoms and overall charge.
What About Significant Resonance Structures?
This is a term you will often hear. Significant resonance structures are the resonance forms that contribute most to the true, overall electronic structure of a molecule. They are the major contributors to the resonance hybrid and closely represent the real electron distribution.
Let’s make it simple: significant resonance structures are those that are correct. So, anything that violates Rules 1 and 2 and their implications is obviously not a significant resonance structure.
However, not everything that doesn’t violate these rules has a significant resonance structure. Just like doing something inappropriate that doesn’t break any rules is still unacceptable.
The first thing that comes to mind is the wrong direction for the flow of electrons. For example, you cannot draw a resonance structure by pushing the electrons of a π bond from the more electronegative atom to the less electronegative atom:
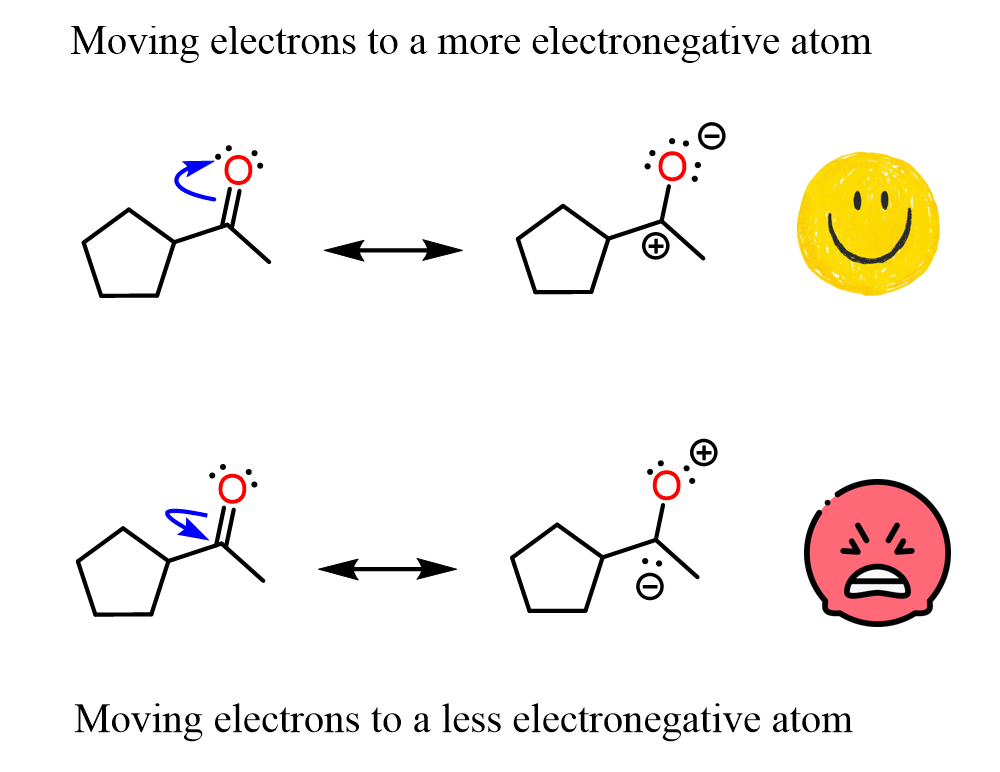
The second example should look wrong to you. Who places a positive charge on oxygen and a negative charge on carbon? This does not violate any of the rules, and structurally it’s not incorrect, but we all know it is wrong, thus it cannot be considered a significant resonance structure.
Summary of Rules for Drawing Resonance Structures
- Resonance structures are different Lewis structures of the same species. Therefore, we do not make or break single bonds when drawing them. Use only lone pairs and/or pi bonds when drawing resonance structures.
- Do not exceed the octet of carbon and second-row elements N, O, F, and the other halogens. Halogens can only have one single bond in organic chemistry. You can have those atoms surrounded by less than 8 electrons as long as you keep track of the number of lone pairs and formal charges. Always pay attention to hidden hydrogen atoms in bond-line structures to avoid exceeding the octet of carbon.
- Do not start curved arrows from a positive charge or the middle of a single bond. Use only lone pairs and/or pi bonds.
Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.
Molecular Representations Quiz