The aldehyde functional group is one of the most common and reactive carbonyl-containing groups in organic chemistry. It plays a central role in oxidation-reduction reactions, nucleophilic additions, and many biological pathways.
It is a carbonyl-containing group, so for starters, recall the central theme of the C=O bond:
Depending on what is connected to the sides of the carbonyl, we have a variety of functional groups. In aldehydes, at least one hydrogen atom must be connected to the carbonyl carbon. The other substituent can be a hydrogen, an alkyl, an aryl, vinyl, or, in general, any carbon chain.
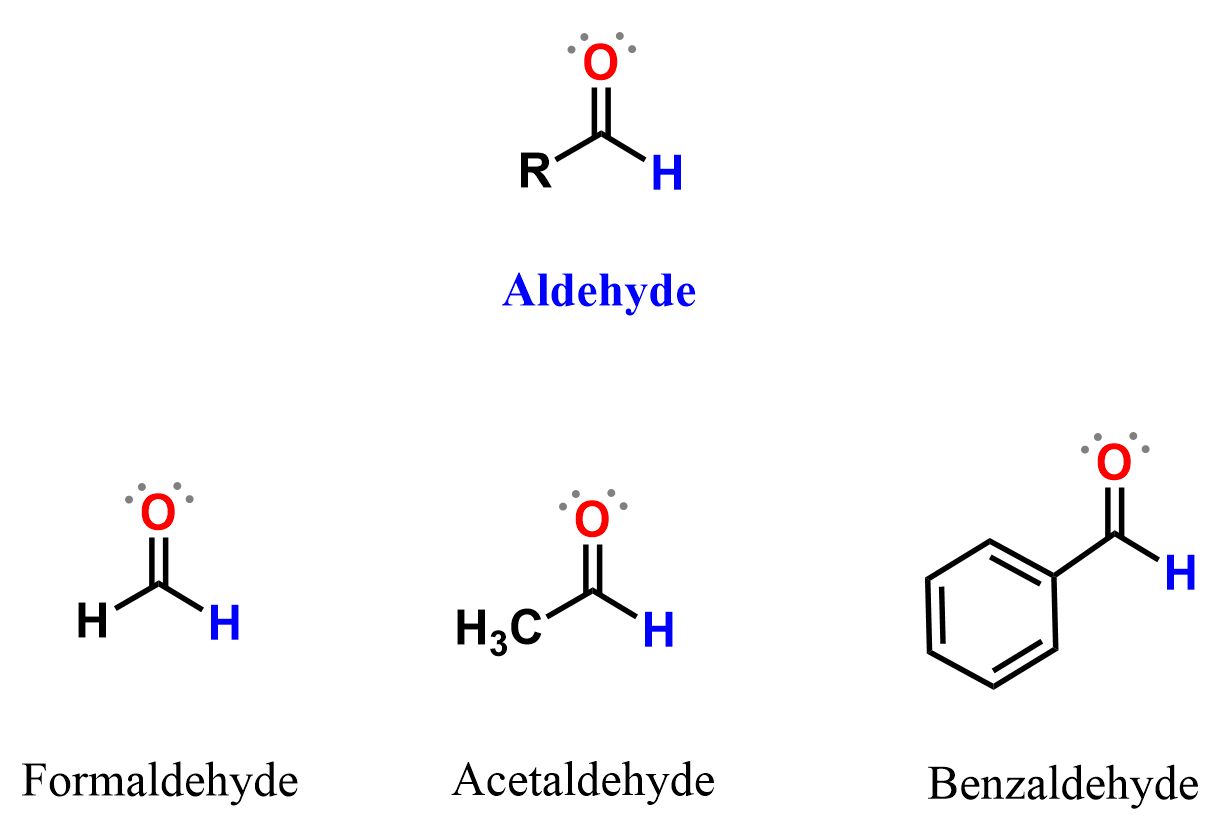
Nomenclature of Aldehydes
The simplest aldehyde is formaldehyde (methanal), which has two hydrogen atoms attached to the carbonyl carbon. The next one in the series is acetaldehyde (ethanal), followed by propionaldehyde (propanal), butyraldehyde (butanal), and so on.
When the carbonyl group is attached to an aromatic ring, the compound is called an aromatic aldehyde, such as benzaldehyde.
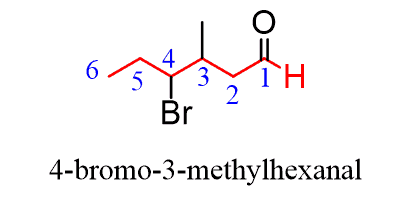
These are common names that are often assigned to the most important representatives of the given functional group. Let’s also briefly cover how they are named according to the IUPAC rules. You need first to find the longest carbon chain containing the -CHO group and change the suffix from “ane” to “al”, dropping the “e” and the locant “1” in the final name:

Everything else is based on the IUPAC nomenclature rules for simple alkanes.
The substituents are placed in alphabetical order:
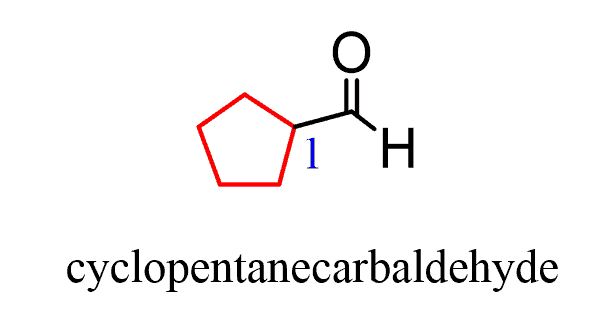
The only out-of-normal situation you may encounter is when the -CHO group is on a ring. In this case, the suffix changes to “carbaldehyde”:
We have a separate post dedicated to the nomenclature of aldehydes and ketones, so check it out for more details, examples, and practice problems.
Geometry and Hybridization in Aldehydes
The carbonyl carbon in an aldehyde is sp² hybridized, forming a planar structure with bond angles of approximately 120°.
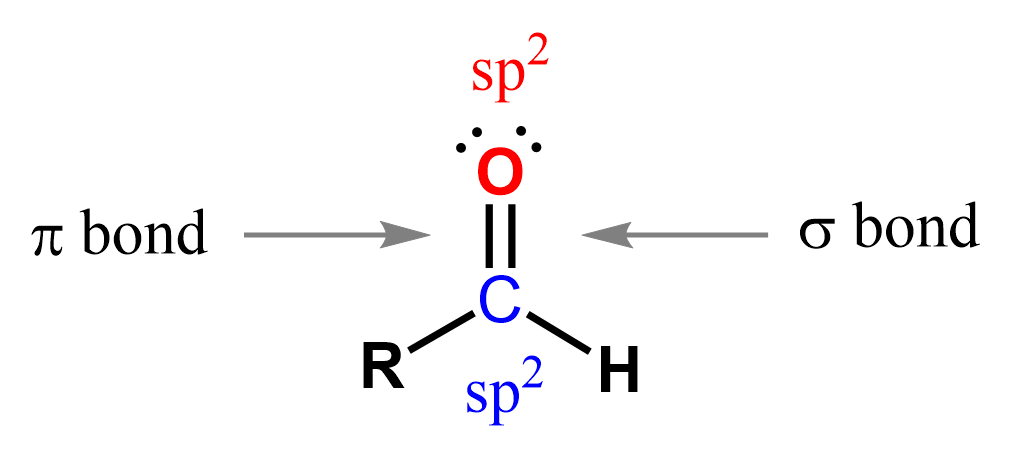
The C=O bond is highly polar, which gives aldehydes and other carbonyl-containing compounds their characteristic reactivity toward nucleophilic addition reactions.
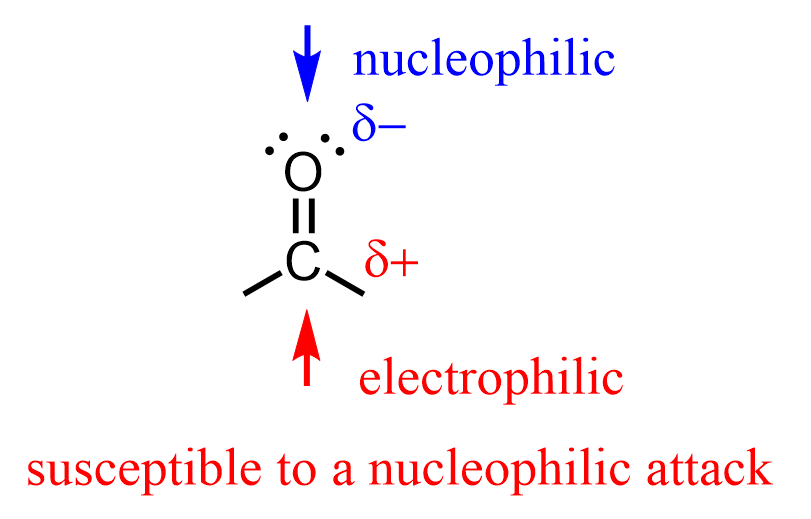
This reactivity is the foundation of the addition-elimination mechanisms, which is an Organic Chemistry II topic, so don’t worry too much about it if you haven’t covered it yet. However, if you’re interested, you can read more about it in the section on nucleophilic addition to carbonyls.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- How to Determine the Number of Lone Pairs
- Bonding Patterns in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis, and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
