The amide functional group is an important carbonyl-containing group in organic chemistry. Amides are widely found in proteins, peptides, pharmaceuticals, and synthetic polymers, and they play key roles in condensation, hydrolysis, and acylation reactions.
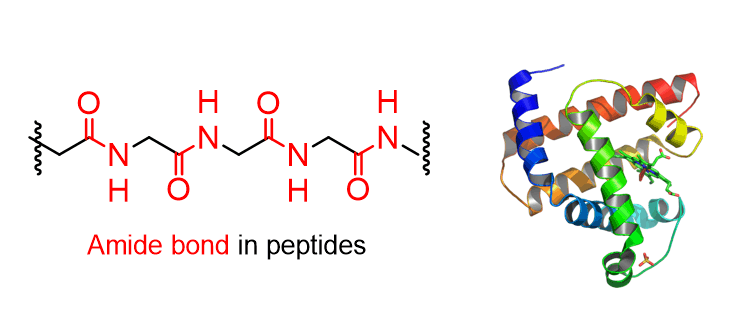
Since amides contain a carbonyl group, the C=O bond remains the central feature of their reactivity.
Amide bonds are very strong and rigid because the nitrogen can share its lone pair of electrons with the carbonyl group. This gives the C–N bond partial double-bond character, which makes the bond shorter, stronger, and less able to rotate. Because of this strong bonding, amides are the least reactive of all carboxylic acid derivatives. This same strength is what makes certain amide-based polymers, called aramids, so special.

Materials like Kevlar and Nomex use these strong amide bonds along with rigid aromatic rings to create fibers that are extremely tough, heat-resistant, and durable, which is why Kevlar is used in bulletproof vests and other high-performance applications.
Structure of Amides
In amides, the carbonyl carbon is bonded to a nitrogen atom (–NR′R″) instead of a hydrogen (as in aldehydes) or another carbon (as in ketones). The general structure of an amide is:
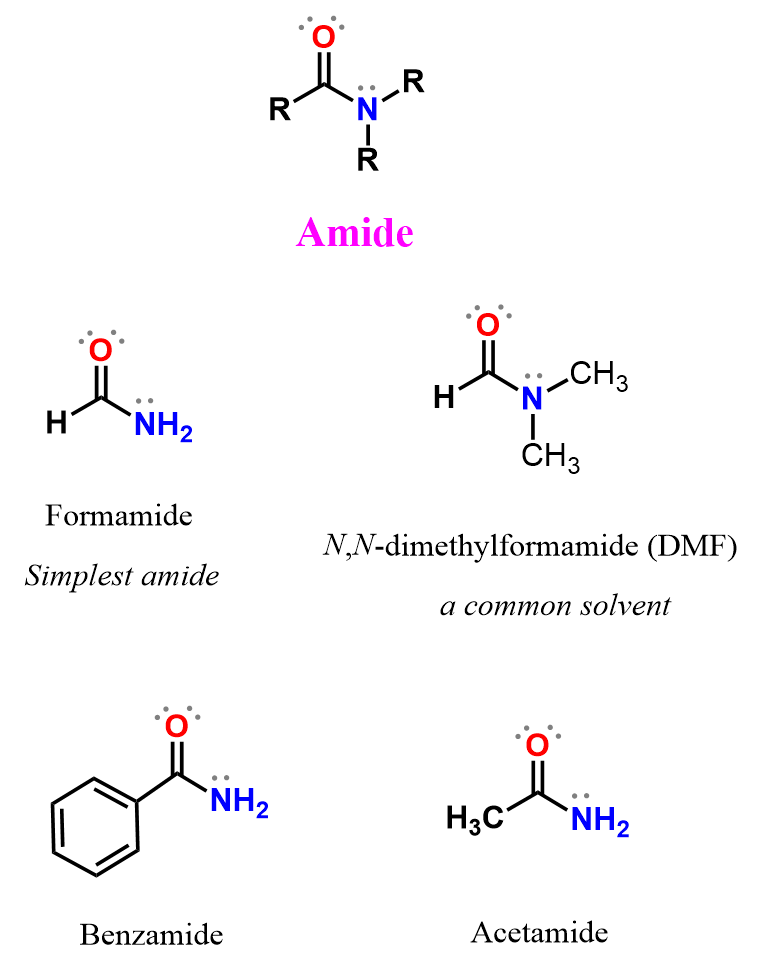
- R is a carbon chain (alkyl or aryl group) attached to the carbonyl carbon.
- R′ and R″ can be hydrogen or carbon groups attached to the nitrogen atom.
- Amides can be viewed as derived from carboxylic acids, where the –OH group has been replaced with an –NR′R″ group.
Depending on the number of alkyl or aryl groups attached to the nitrogen atom, amides are classified as primary, secondary, or tertiary:

- Primary amides (R–CONH₂) – the nitrogen is bonded to two hydrogens.
- Secondary amides (R–CONHR′) – the nitrogen is bonded to one hydrogen and one alkyl/aryl group.
- Tertiary amides (R–CONR′R″) – the nitrogen is bonded to two alkyl or aryl groups and no hydrogens.
The number of alkyl groups connected to the nitrogen affects the reactivity, hydrogen bonding, and physical properties of amides.
Lactams – Cyclic Amides
A special type of amide is a lactam, which is a cyclic amide formed when a hydroxyl and an amino group within the same molecule react, eliminating water. Recall that cyclic esters were named lactones:
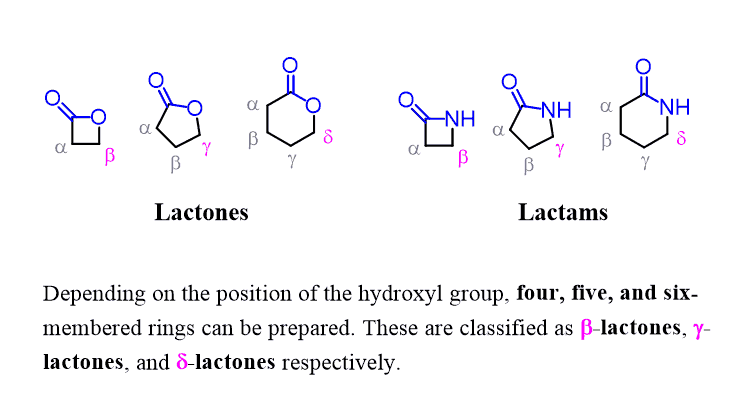
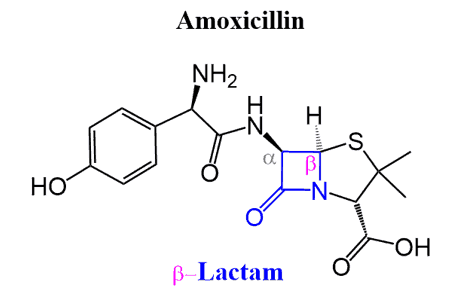
Lactams are common in nature and in pharmaceuticals, such as β-lactams, which are found in penicillin. Their names are based on the ring size and the position of the amide linkage—for example, β-lactam (four-membered ring) and γ-lactam (five-membered ring).
For example, amoxicillin is a β-lactam antibiotic, meaning it contains a four-membered cyclic amide (lactam) ring. This strained ring is highly reactive, which allows the drug to inhibit bacterial cell wall synthesis, making it effective against a wide range of bacterial infections.
Preparation of Amides
Amides are most commonly prepared through a condensation reaction between a carboxylic acid (or derivative) and an amine. In this reaction, the nucleophilic amine attacks the carbonyl carbon, and water is eliminated to form the amide.
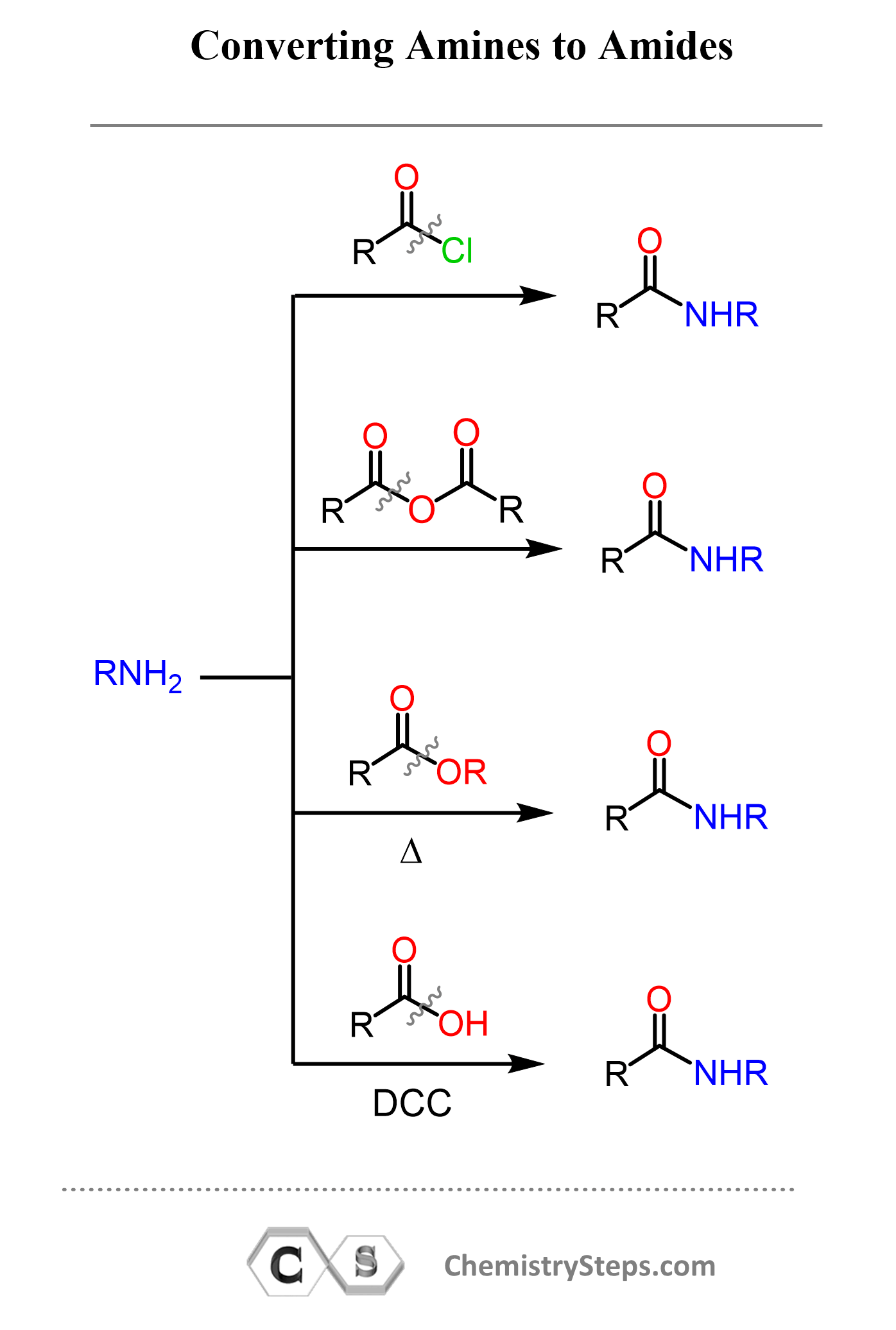
As shown above, amides can also be synthesized from acid chlorides or anhydrides reacting with amines. These reactions are faster and more complete because acid derivatives are more reactive than carboxylic acids. Another method involves direct acylation of amines in the presence of coupling agents, which is especially useful in peptide synthesis.
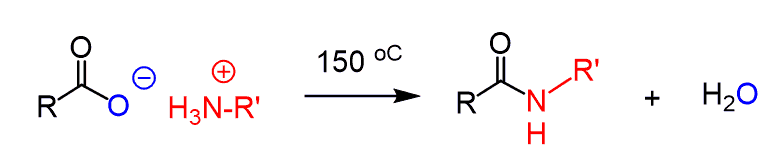
It is worth mentioning that Amines can also react with carboxylic acids to form amides. However, the –OH group is not a good leaving group, and therefore, the direct conversion requires high temperatures (usually >150 °C), where not all molecules can survive.
To perform this conversion under milder conditions, a coupling agent such as DCC (N,N′-dicyclohexylcarbodiimide) or EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide) can be used. Both are carbodiimides (RN=C=NR), which prevent the acid-base reaction and make the carboxylic acid susceptible to nucleophilic attack.
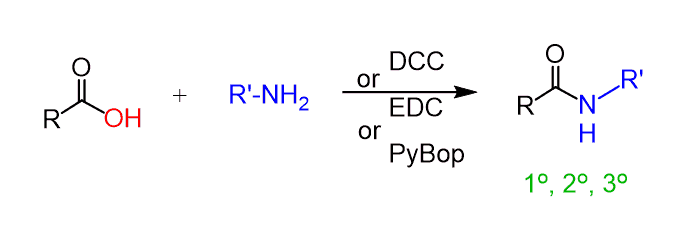
We have a separate post dedicated to the reaction between amines and carboxylic acids using different coupling reagents, so feel free to check that out as well.
Nomenclature of Amides
Amides are derivatives of carboxylic acids, and if you have already read about the nomenclature of carboxylic acids, you are familiar with the suffixes -ic acid, -oic acid. Now, for primary amides, all you need to do is replace the -ic acid, or -oic acid ending with the suffix “amide”.
For example:

All the substituents are numbered by starting from the amide carbon (unless a higher priority group is present) and placed alphabetically, just like for naming any other functional group:

When the amide group is connected to a ring, the suffix “carboxylic acid” is replaced with “carboxamide”:
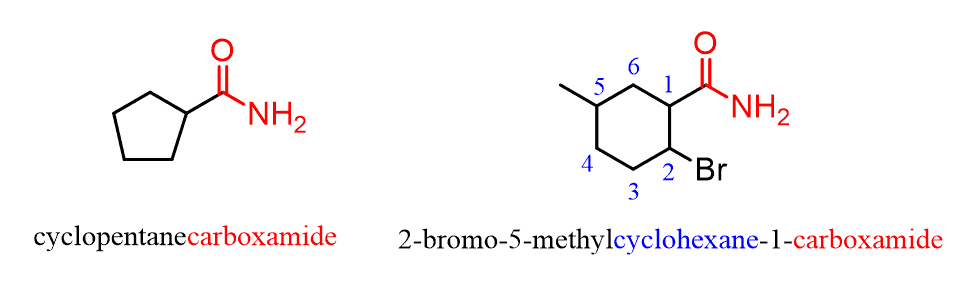
Notice that the amide carbon, in this case, is not counted as part of the parent chain.
We have a separate post on the nomenclature of amides, so feel free to check it for more details and examples.
Geometry and Hybridization in Amides
The carbonyl carbon in amides is sp² hybridized, forming a trigonal planar geometry. The carbonyl oxygen is also sp² hybridized. Like the OR group in esters, the nitrogen atom exhibits characteristics of both sp³ and sp² hybridization because one of its lone pairs can delocalize into the carbonyl group through resonance, creating partial double-bond character between the carbon and nitrogen.

This delocalization partially flattens the nitrogen geometry and strengthens the C–N bond. You can read more about localized and delocalized electrons in this post, and we also have a separate article explaining how lone pairs affect hybridization if you want more details.

Reactivity of Amides
Amides, like aldehydes, ketones, and esters, contain a polar C=O bond, making the carbon electrophilic. However, the nitrogen lone pair donates electron density into the carbonyl, reducing its electrophilicity.
Amides are therefore the least reactive among carboxylic acid derivatives. They are resistant to nucleophilic attack under mild conditions, which is why hydrolysis usually requires strong acids or bases and heat.
Here is a summary of the most important reactions of amides:

Once again, the preparation and reactions of amides are covered in Organic Chemistry 2. In Organic Chemistry 1, the focus is on recognizing and understanding their structure and basic properties.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- How to Determine the Number of Lone Pairs
- Bonding Patterns in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis, and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
