In the previous post, we talked about the intermolecular interactions, their relative strengths, and correlation to the boiling and melting points of organic compounds.
Using the chart with the order of intermolecular interactions, try to solve the following practice problems on the boiling and melting points:




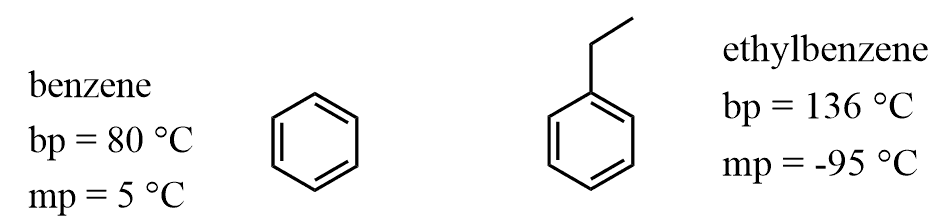
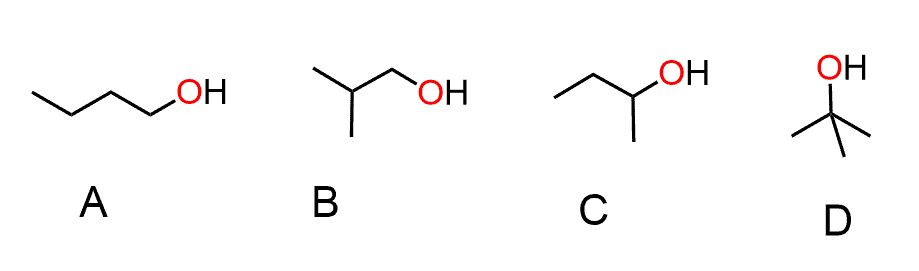
Question #2 in C,
How can B option make 2 Hydrogen bonds?
There are two oxygens so both can be involved:
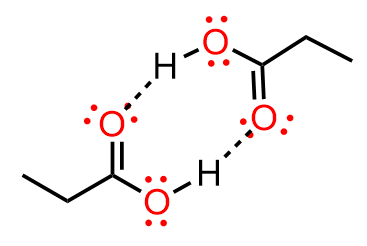
AHHH
I got it !
It’s easier to understand with the figure.
Thank you
No worries. Feel free to ask your questions.
Hi, I’m a bit confused about 1d. Why does the answer mention boiling point when the question asks for melting point?
I understand that A would have a higher boiling point because the lack of branching increases surface area, and D would have a higher melting point because it is symmetrical?
Thanks!!
Sorry, the first question must be on boiling point, and your reasoning is all correct.
I have also added the values in the Physical Properties study guide which you can download here.
Thanks for bringing it up!