Enantiomers and diastereomers are types of stereoisomers. Recall that stereoisomers are isomers with the same connectivity but different spatial arrangement of atoms. The isomers with different connectivity of atoms are called constitutional isomers:

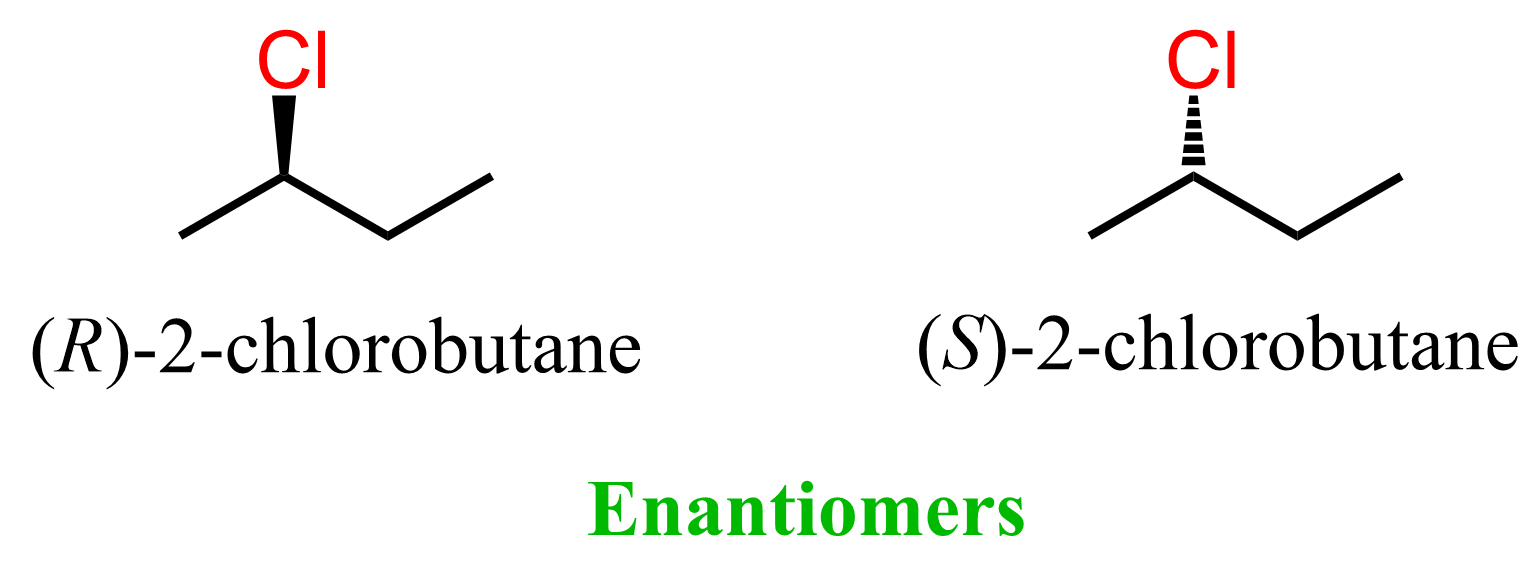
The (R)-2-chlorobutane and (S)-2-chlorobutane shown above are examples of stereoisomers because, even though the atoms are connected identically, the chlorines are pointing in different directions.
One chlorine is represented with a wedge bond, and the other with a dashed line. Importantly, we cannot just flip the molecules and change the wedge dash notation, keeping the connectivity intact. Flipping the molecule 180o. changes the position of the Cl and even though it is a dash now, the overall representation of the molecule is different which we can confirm by numbering and naming the molecules:
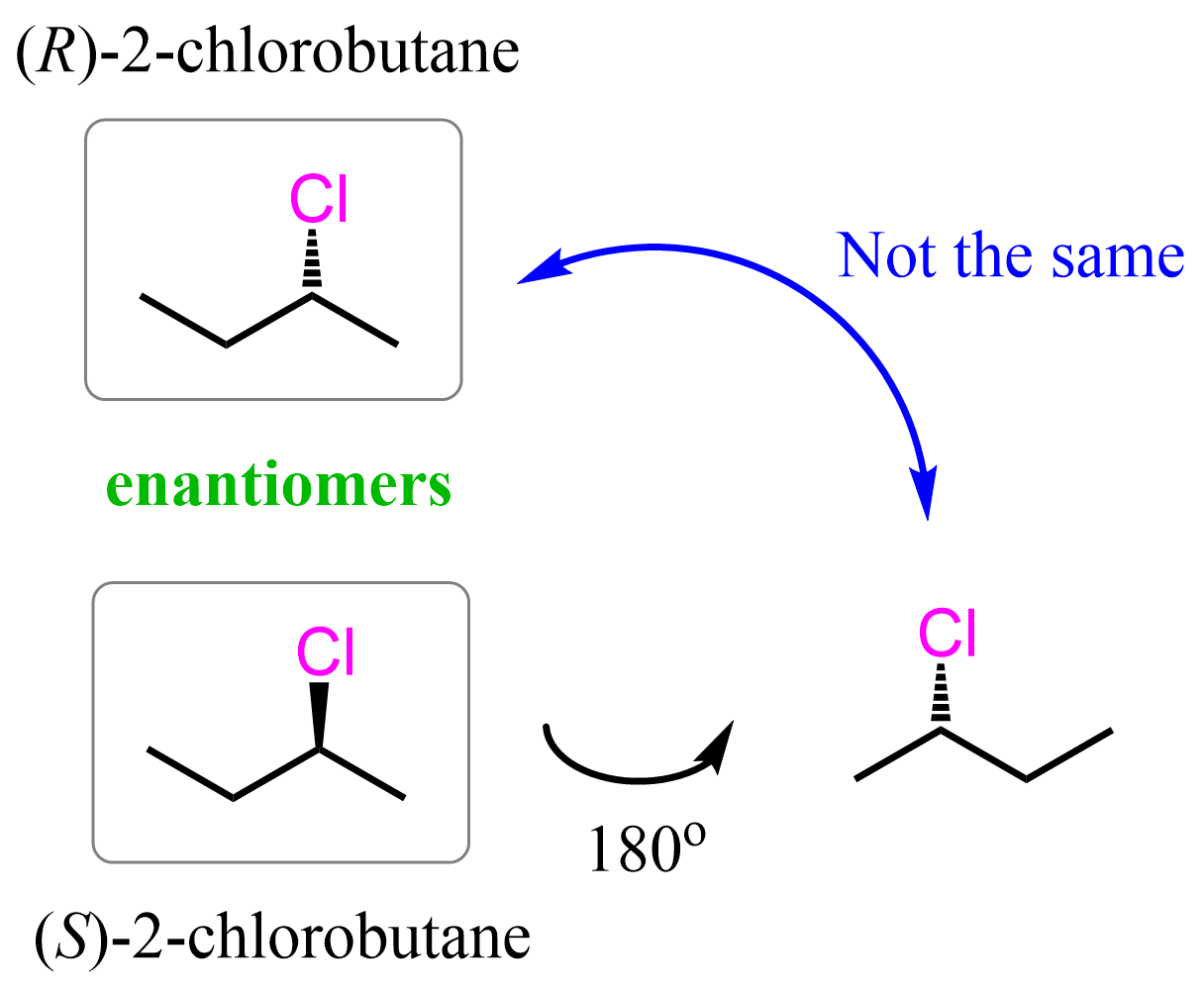
This means the molecules are nonsuperimposable mirror images which are known as enantiomers. A reminder that superimposing molecules can be repeated in space even though they may be represented differently. As an example, consider these two representations of dichloromethane:
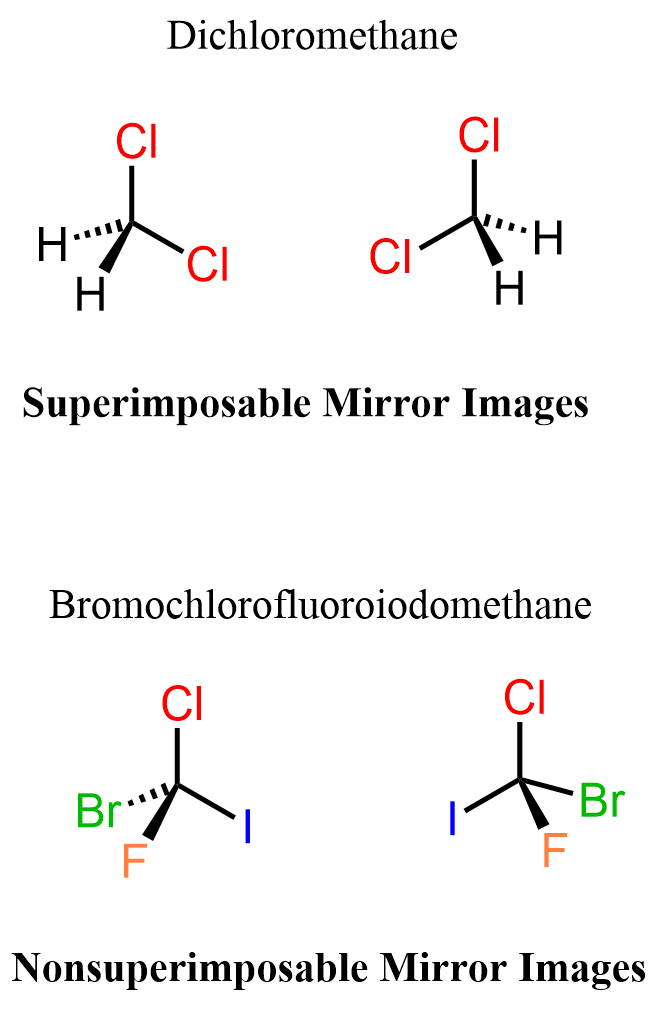
The first pair is two representations of dichloromethane which are mirror images. The mirror images, however, are superimposable which means they represent the same molecule:
On the contrary, if the carbon atoms had four different atoms connected it, the mirror image wouldn’t be superimposable to it – they would’ve been enantiomers:
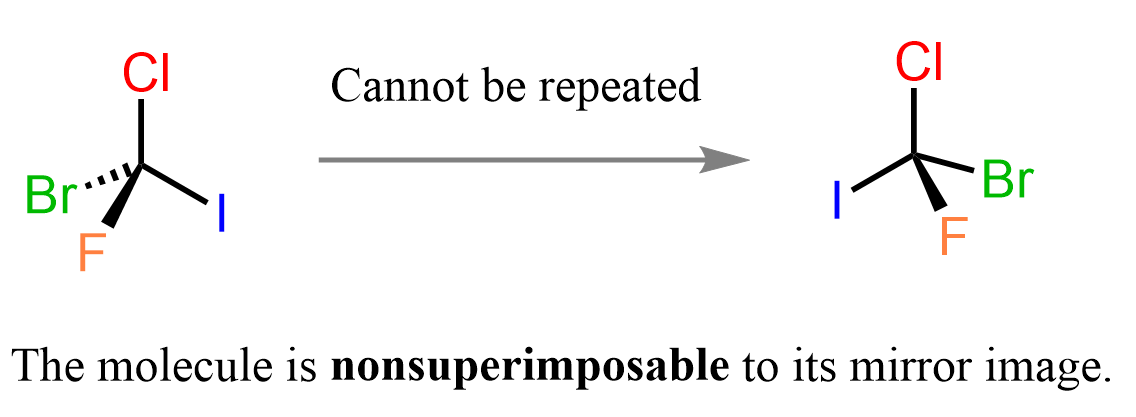
If you feel like this is some kind of trick and the objects are the same, try building the two molecules using a model, and you will see that you won’t be able to repeat identical representations – they are not superimposable.
The same is true about (R) and (S)-2-chlorobutane – they are nonsuperimposable mirror images, and therefore, enantiomers.
So, let’s define at this point that enantiomers are stereoisomers that are nonsuperimposable images of each other.
Any object that is non-superimposable to its mirror image is said to be Chiral. In chemistry, the origin of chirality is most often a carbon(s) with four different groups present in the molecule. This is also known as a chiral or chirality center which is a type of a stereo- or stereogenic center:
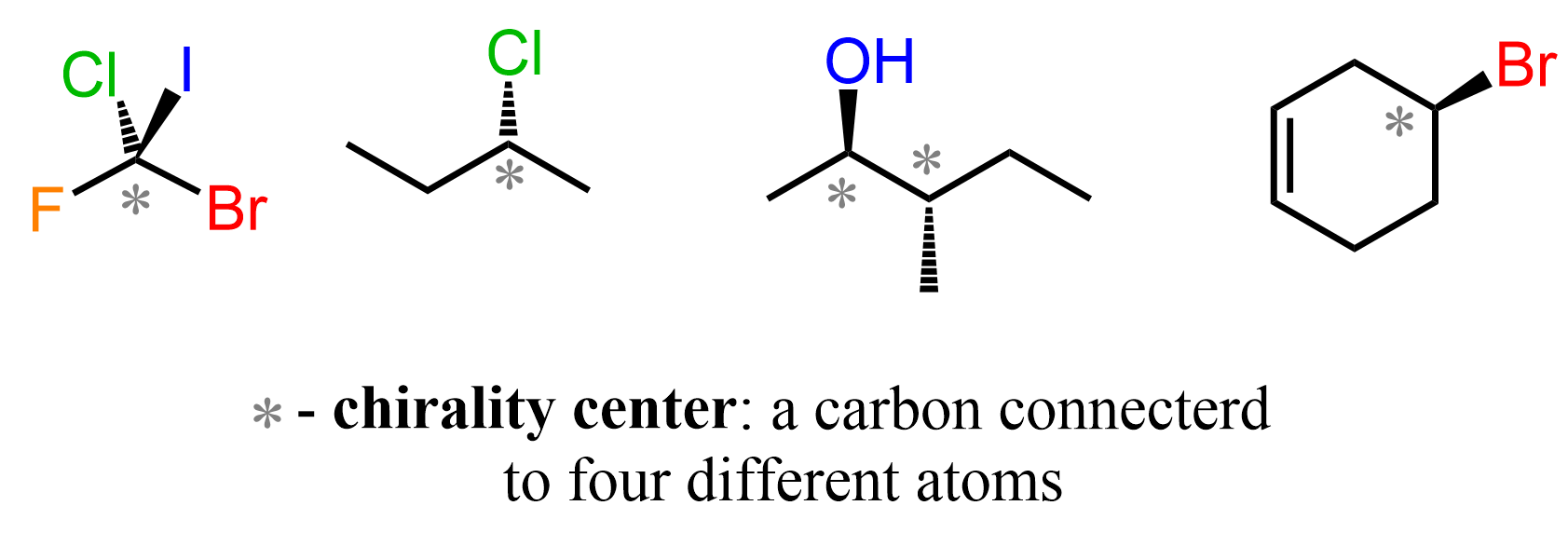
So, most often, to be chiral the molecule must contain at least one chirality center – that is a carbon with four different groups connected to it.
How to Draw the Enantiomer of a Molecule
You can draw the enantiomer of a molecule by placing a mirror on any side of it. There can be an infinite number of mirror images of a chiral molecule but they all represent the same molecule – its enantiomer. Remember, a molecule has only one enantiomer. As an example, consider the following three molecules and their enantiomers drawn differently based on the angle of the mirror placed next to it:
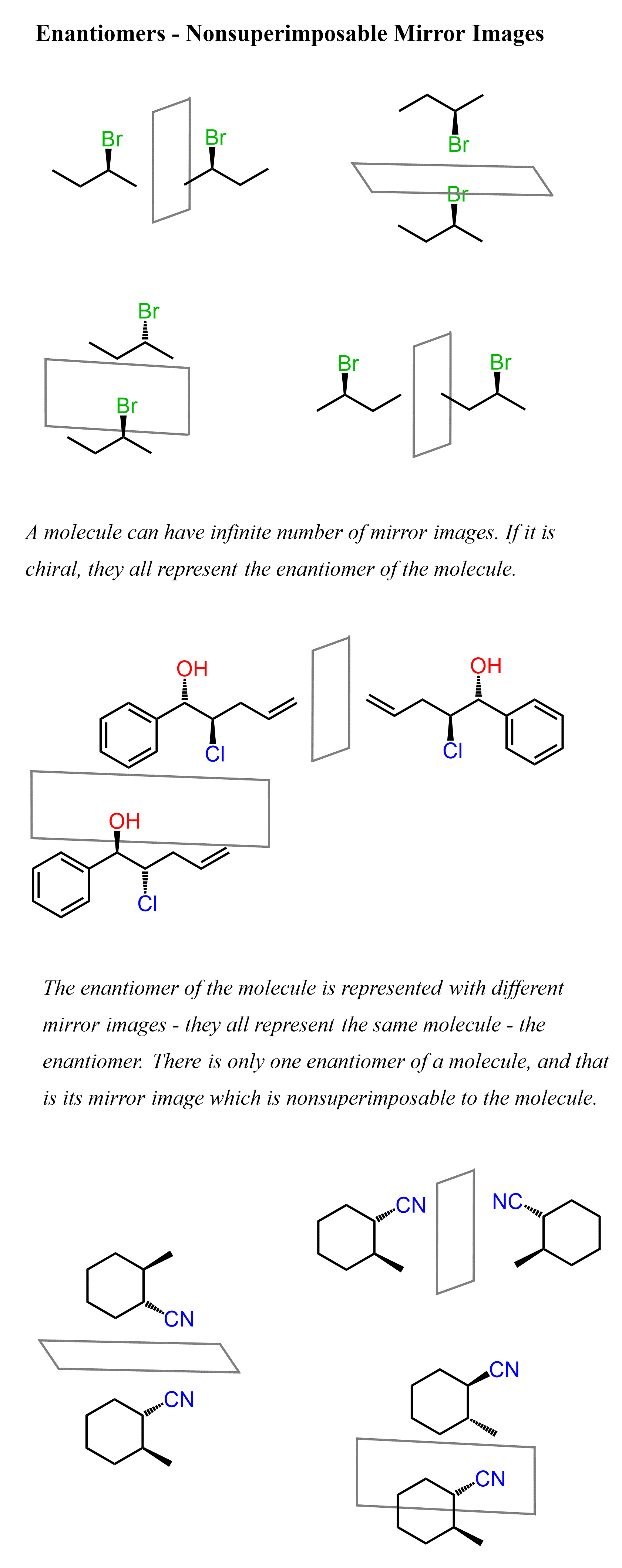
Let’s mention at this point that you do not have to place a mirror to draw the enantiomer of the molecule. This was just to illustrate the correlation between enantiomers and mirror images.
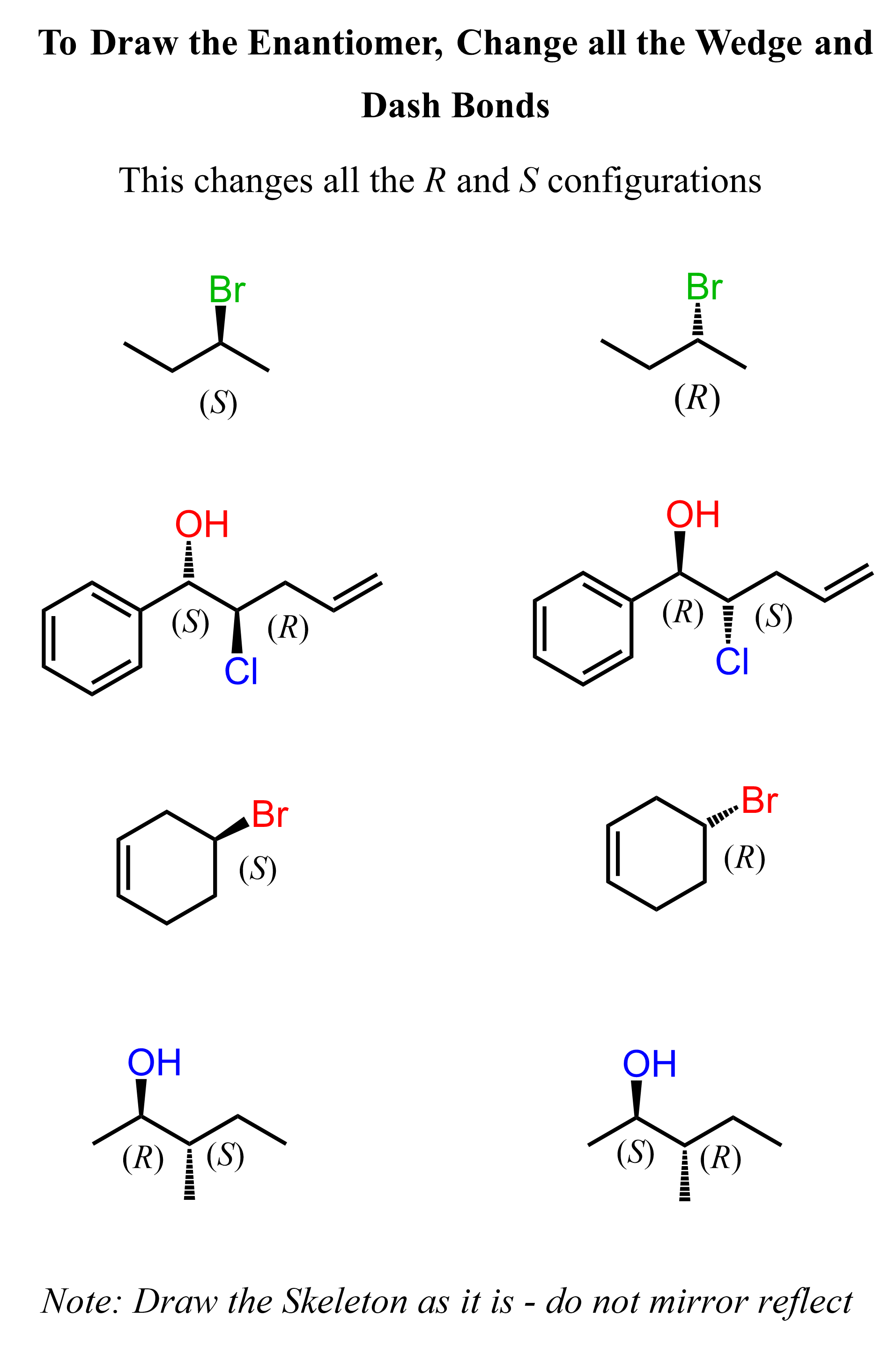
The most common strategy for drawing enantiomers is to switch every wedge and dash in the molecule, which changes the absolute configuration of those chiral centers. Remember, wedge and dash are relative to the view angle, and that is why we have the R and S notation for absolute configuration:

To draw the enantiomer of (R)-2-chlorobutane shown above, we simply change the chlorine from wedge to dash, and it becomes (S)-2-chlorobutane:
Let’s emphasize at this point that all the chiral centers of enantiomers are inverted – every R is inverted to S and every S is inverted to R.
So, let’s draw the enantiomers of the three molecules we did earlier by simply switching all the wedges and dashes without the use of a mirror:
Notice that for the first two molecules, the particular representation of the enantiomer was also obtained when we used the method with a mirror. Once again, all the mirror images represented the same molecule – the enantiomer of the original molecule. There can only be one enantiomer for any chiral molecule.
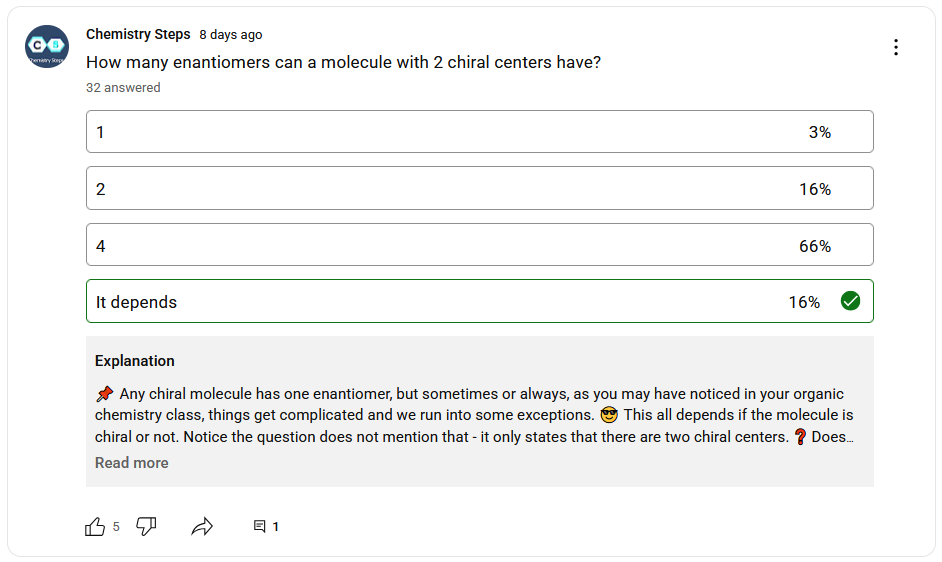
This is a quiz from 🔗our YouTube channel, and it screams to be here to remind us all that whenever we say only/always etc. in organic chemistry, there is always an exception to that 🤦♂️.
What do you think, how many enantiomers does a molecule with two chiral centers have?
Going back to drawing enantiomers!
An important reminder here about a mistake that I have seen many students make! When drawing the enantiomer of the molecule, you can either switch all wedge and dash representations of the chiral centers, or you can draw the mirror reflection of the molecule on the sides, on the top, or below, and keep the wedge and dash notation as it is. The wedge and dash notation is only changed when the mirror is placed behind or in front of the molecule because the zig-zag (skeleton) of the molecule is not changed in this case.
You should not do both: you should not draw the mirror reflection and change the wedges and dashes together with it, since you will end up having the same molecule. As an example of this mistake, we first did the reflection of the molecule and then changed the wedge and dash notation, and instead of the enantiomer, we got the same molecule, only flipped at 180o:

Alright, enough of enantiomers – they are types of stereoisomers, they are chiral, and they are nonsuperimposable mirror images. Let’s now see what diastereomers are.
Diastereomers
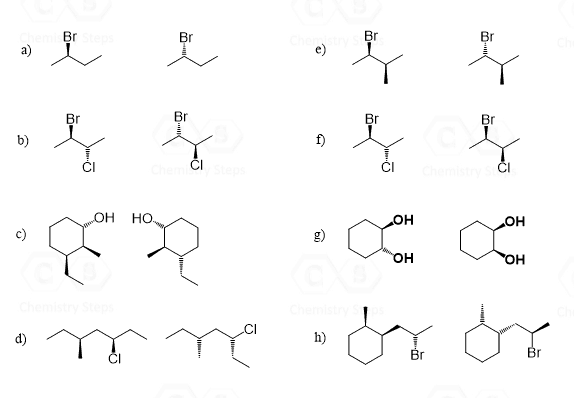
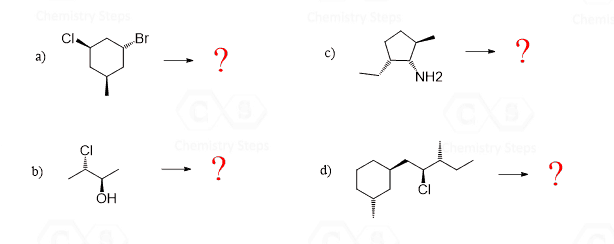
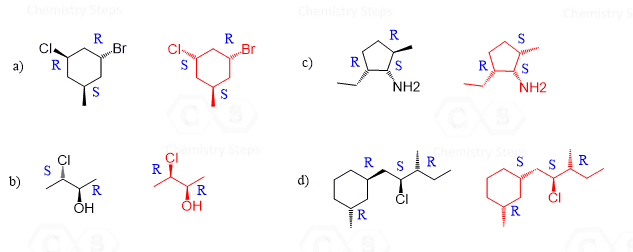
Diastereomers are stereoisomers that are not mirror images. Although it may sound so, this definition is not for lack of a better word – it is the official definition of diastereomers. To see this and find out if we could come up with a better definition, let’s draw the diastereomer(s) of the molecules we have been discussing so far.
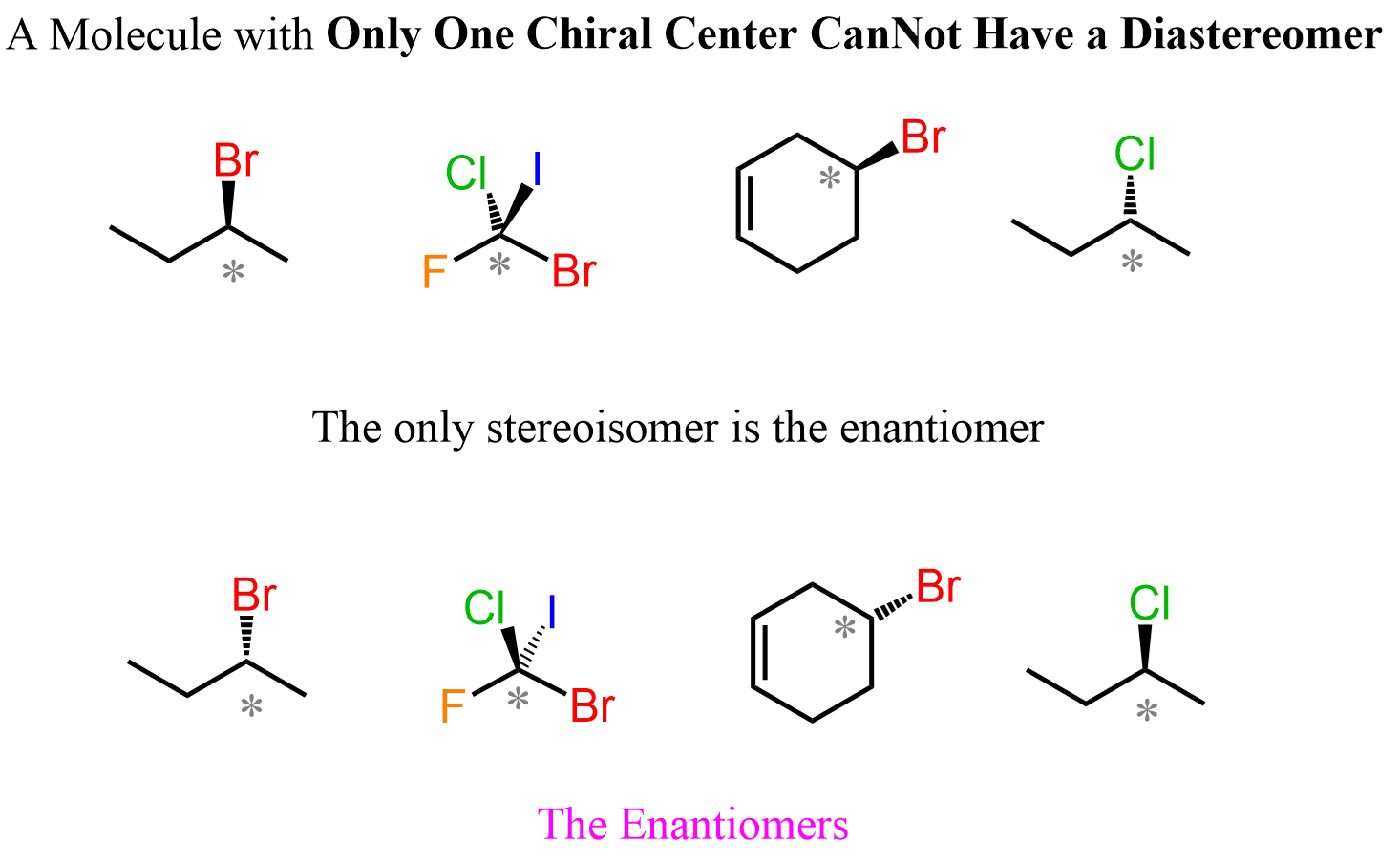
Let’s mention right away that a molecule with one chiral center cannot have a diastereomer, as no matter how we try to change the orientation of the atoms, we’ll end up with the same stereoisomer that is the enantiomer of the molecule. So, bromochlorofluoroiodomethane and 2-bromobutane exist only in two stereoisomeric forms, which are enantiomers:
For all the other molecules with two or more chirality centers, the R and S configurations are inverted in any combination except when all of them are:

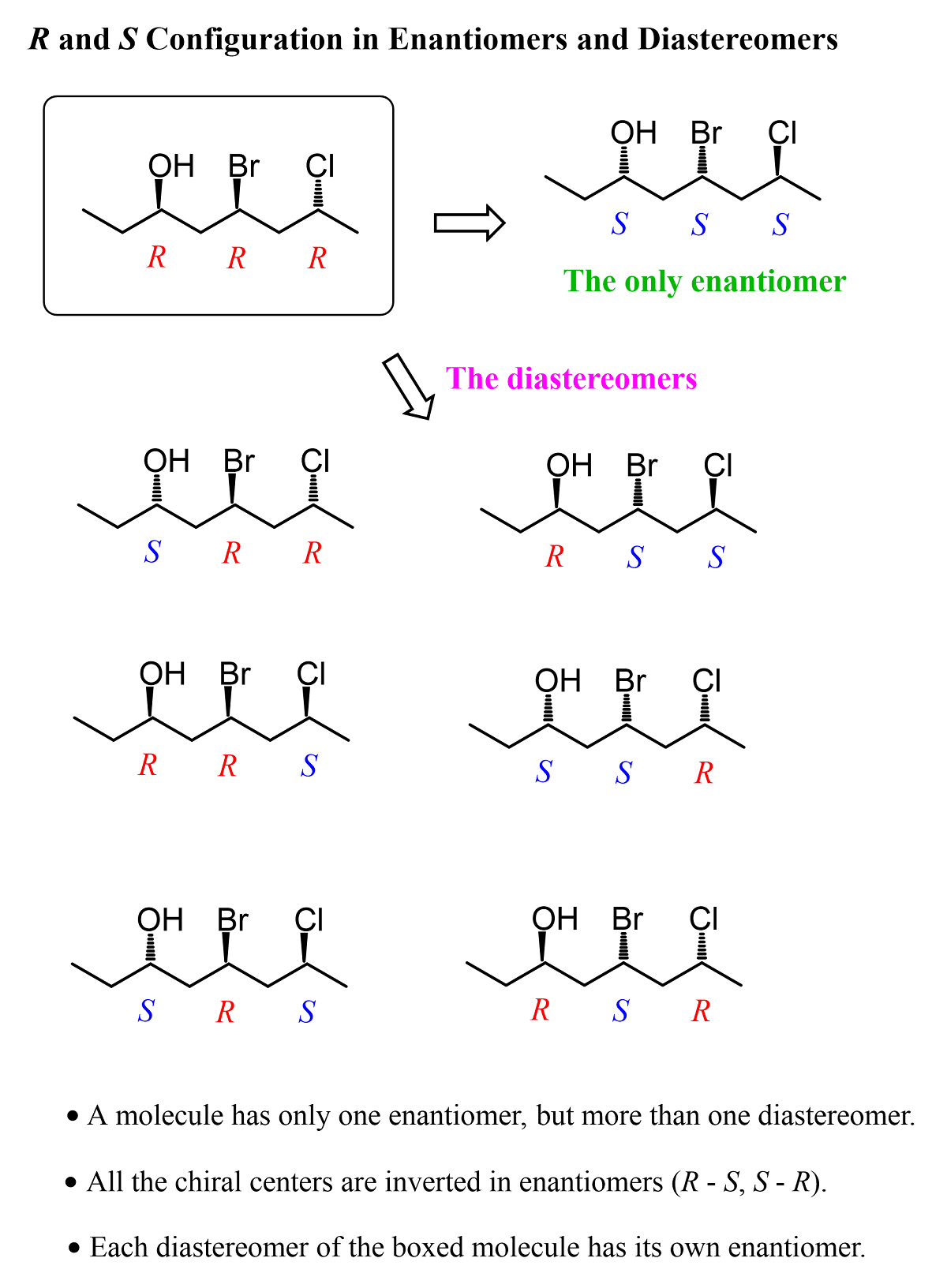
So, in diastereomers, some of the chiral centers are inverted and some are not. This can be one inverted and the rest retained, or vice versa – all inverted and one retained. Let’s also consider a molecule with three chiral centers. Suppose they all have an R configuration:
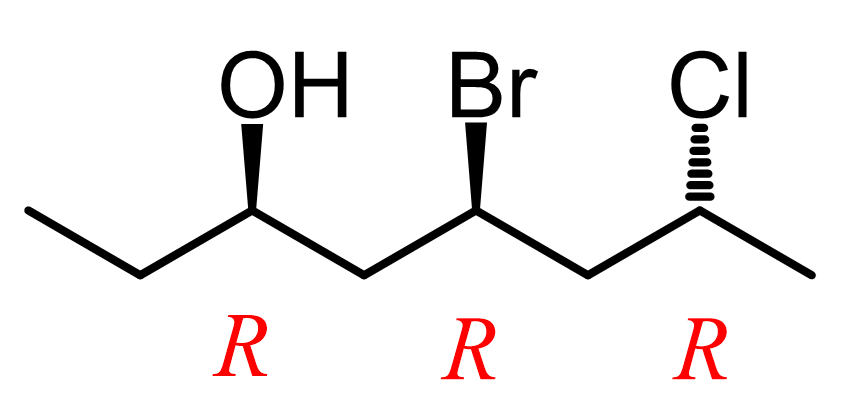
To draw the enantiomer, we keep the zig-zag as it is, and change all the wedges to dashes. This changes every R to S. To draw the diastereomers of the molecule, we draw all the wedge and dash combinations except when they are all the same or all inverted:
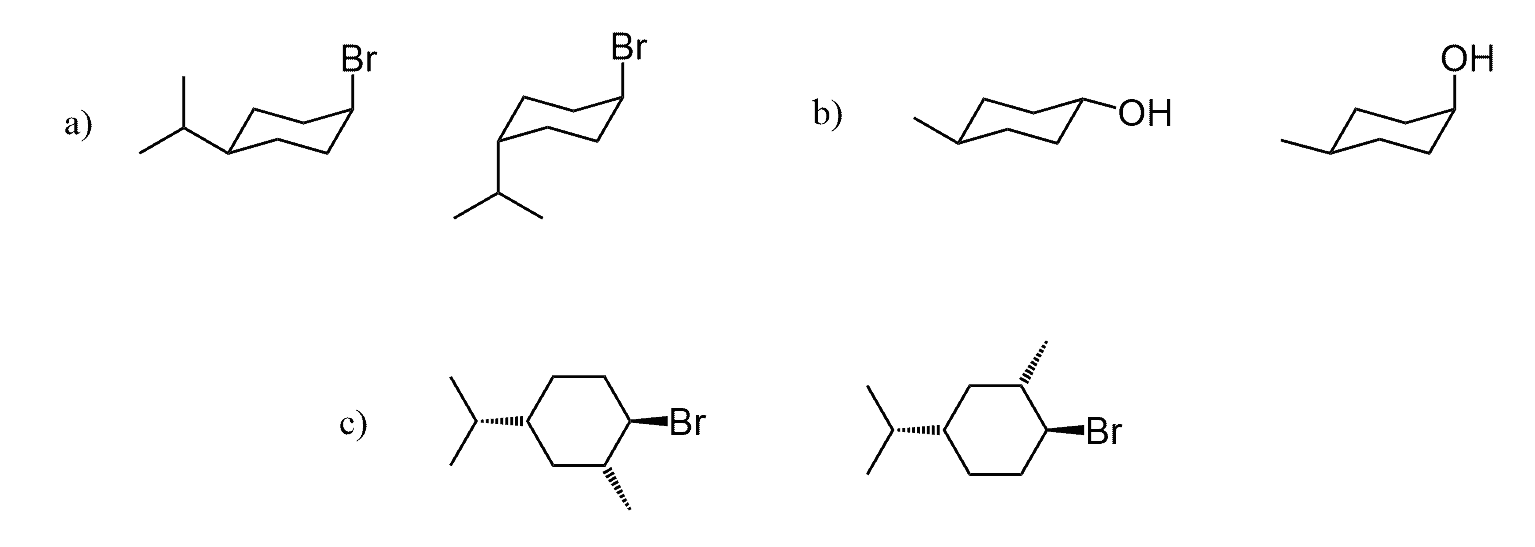
Achiral Diastereomers
The definition of diastereomers covers a broader range of stereoisomers as they are not mirror images. Because of this, achiral molecules can also be diastereomers. For example, cis and trans or E and Z isomers are diastereomers.

Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers.