The ester functional group is another common and important carbonyl-containing group in organic chemistry. Esters are widely found in fragrances, flavorings, fats, oils, and biological molecules, and they play key roles in condensation and hydrolysis reactions.
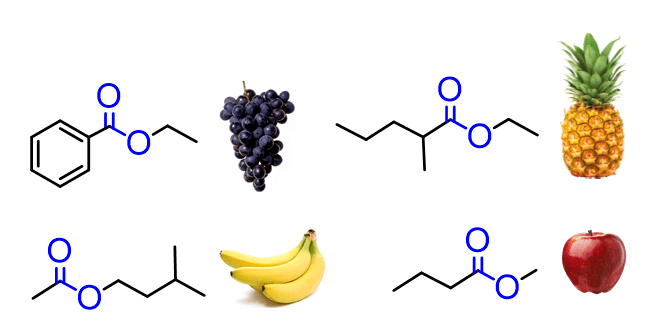
Since esters contain a carbonyl group, the C=O bond remains the central feature of their reactivity.
Structure of Esters
In esters, the carbonyl carbon is bonded to an alkoxy group (–OR′) instead of a hydrogen (as in aldehydes) or another carbon (as in ketones). The general structure of an ester is:
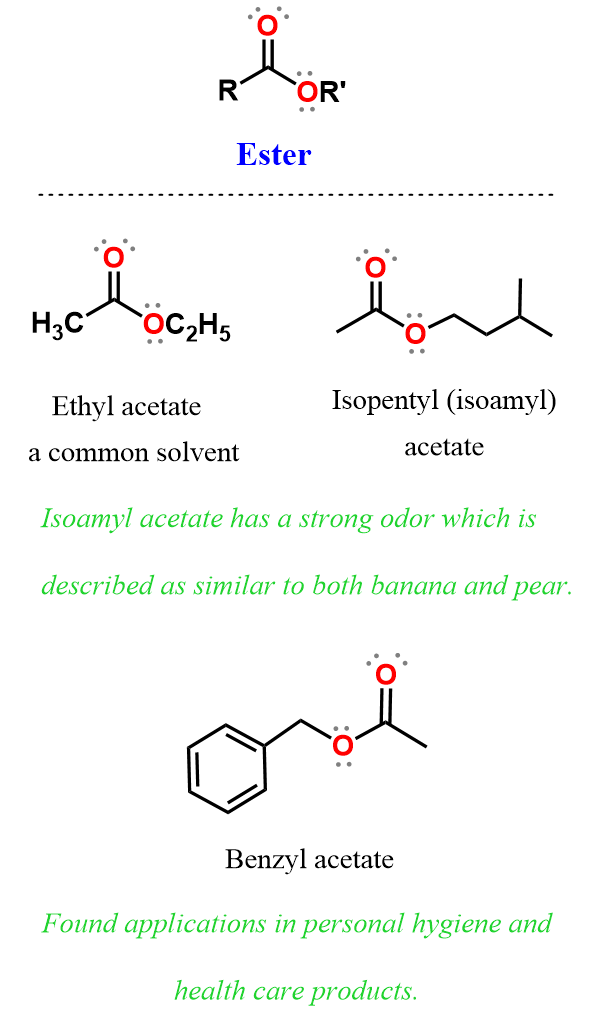
- R is a carbon chain (alkyl or aryl group) attached to the carbonyl carbon.
- R′ is the alkyl or aryl group bonded to the oxygen atom of the -OR′ substituent.
- Esters can be viewed as derived from carboxylic acids, where the -OH group has been replaced with an -OR′ group.
A special type of ester is a lactone, which is a cyclic ester formed when a hydroxyl and a carboxyl group within the same molecule react, eliminating water.
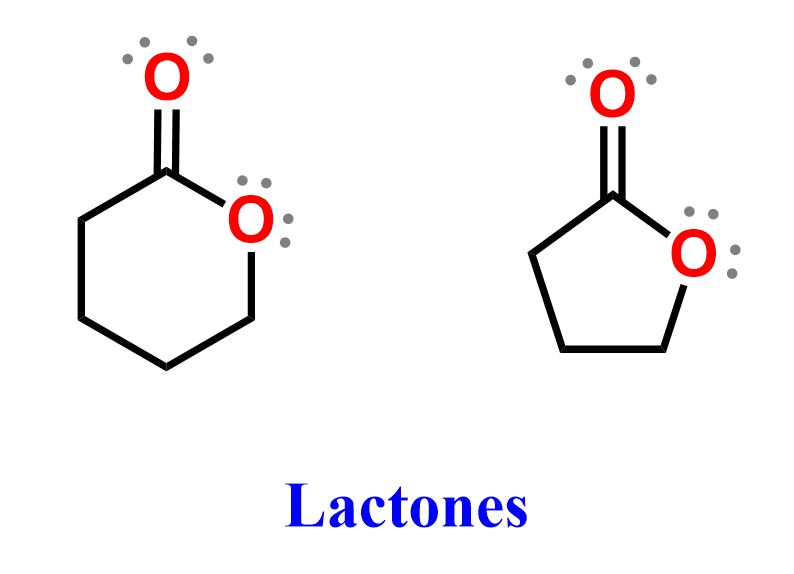
Lactones are common in nature and often contribute to the aroma and flavor of fruits. Their names are based on the ring size and the position of the ester linkage, for example, γ-butyrolactone (five-membered ring) and δ-valerolactone (six-membered ring).
Preparation of Esters
Esters are most commonly prepared through a condensation reaction between a carboxylic acid and an alcohol, known as Fischer esterification. In this reaction, the acid and alcohol are heated in the presence of an acid catalyst (typically concentrated sulfuric acid), which promotes the nucleophilic attack of the alcohol oxygen on the carbonyl carbon of the acid. The reaction produces an ester and water as by-products. For example, reacting acetic acid with ethanol yields ethyl acetate and water.

Esters can also be synthesized from acid chlorides or acid anhydrides, reacting with alcohols, which are often faster and more complete reactions since these derivatives are more reactive than carboxylic acids. Another important method involves an SN2 reaction between a carboxylate ion (RCOO⁻) and a primary alkyl halide (R′–X). In this case, the nucleophilic oxygen of the carboxylate attacks the electrophilic carbon of the alkyl halide, displacing the halide ion and forming the ester.
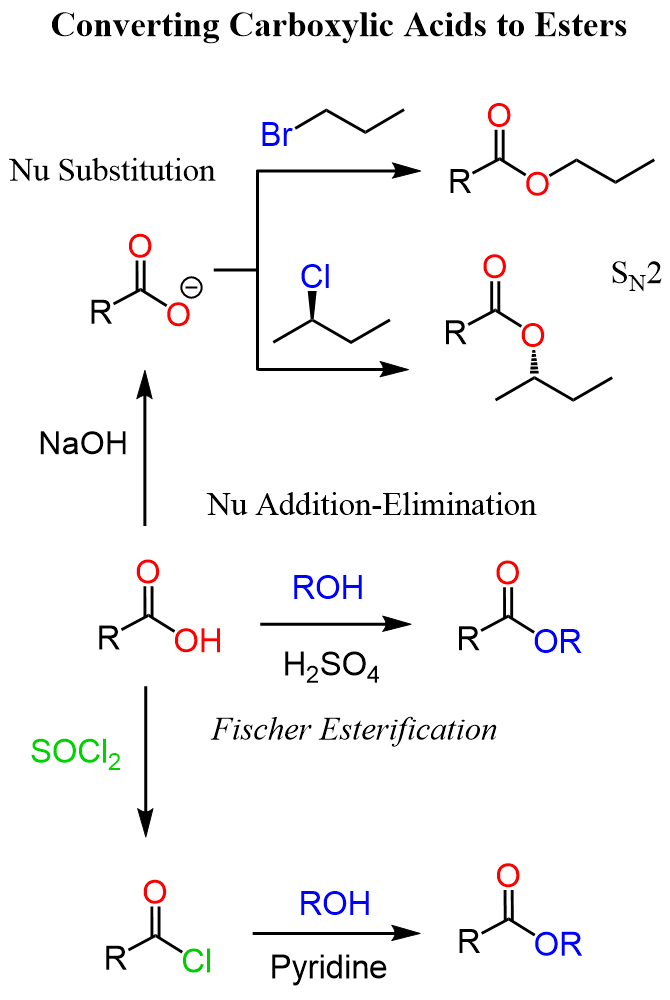
The reaction between carboxylate ions and alkyl halides is known as the alkylation of carboxylate salts, is especially useful when the alcohol is not stable under acidic conditions required for Fischer esterification.
This is an Organic 2 topic, so don’t worry if you’re only starting to learn about functional groups-you’ll study ester reactions and mechanisms later in more depth.
Nomenclature of Esters
In the IUPAC nomenclature of carboxylic acids, we learned that their salts are named by replacing the suffix “ic acid” or “oic acid” with “ate”. For example, sodium acetate, potassium butyrate, etc.
The good news is that esters follow the same pattern, and instead of the metal ion, we use the alkyl group connected to the RCO (acyl) fragment.
For example:

The substituents are numbered based on the position of the COOR group and placed in alphabetical order:

Check the post on the IUPAC rules and nomenclature of alkanes to review these principles. We also have a separate post on the nomenclature of esters, so feel free to check it for more details and examples.
Geometry and Hybridization in Esters
The carbonyl carbon in esters is sp² hybridized, forming a trigonal planar geometry with bond angles close to 120°.
The ester contains two oxygen atoms, each with a different bonding environment. The carbonyl oxygen is sp² hybridized because it is part of the C=O double bond and lies in the same plane as the carbonyl carbon. The alkoxy oxygen (the one in the –OR′ group) shows characteristics of both sp³ and sp² hybridization. This is because one of its lone pairs can delocalize into the carbonyl group through resonance, creating partial double-bond character between the carbon and oxygen.
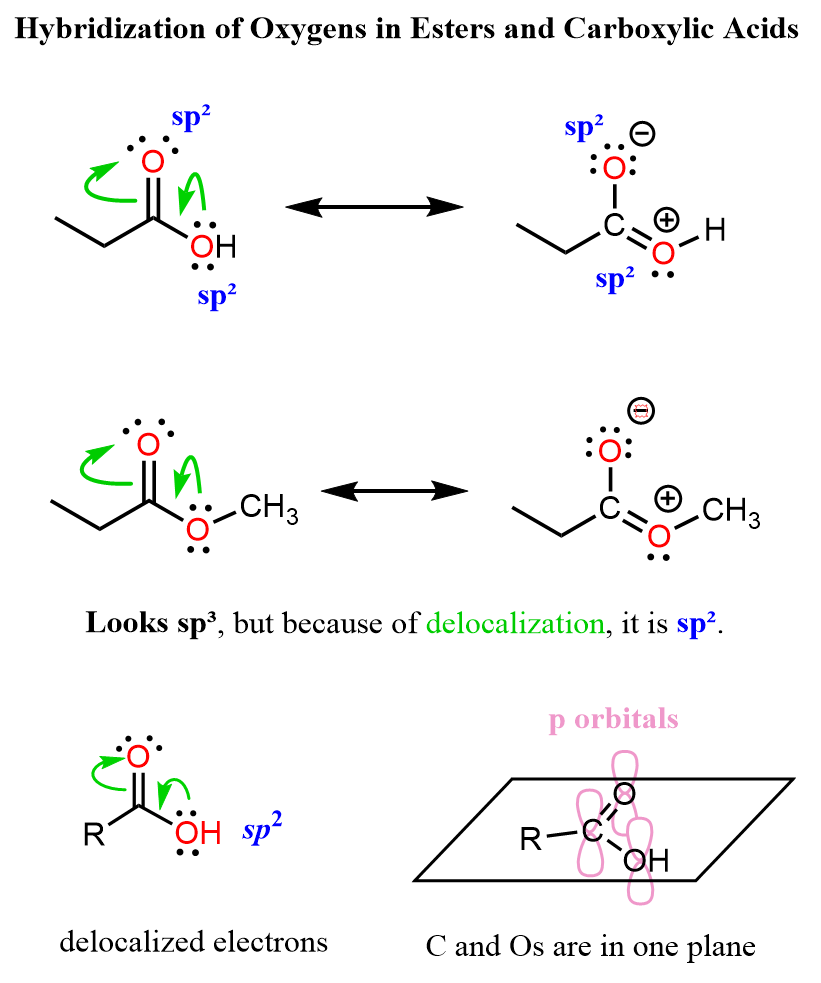
As a result, this oxygen does not fit perfectly into the localized sp³ model – it participates in electron delocalization that gives the C–O bond a shorter and stronger character.
You can read more about localized and delocalized electrons in this post, and we also have a separate article explaining how lone pairs affect hybridization if you’d like to understand this concept in more detail.
Reactivity of Esters
We have seen in the posts about aldehyde and ketone functional groups that the polar nature of the C=O bonds makes the carbon electron-deficient and thus reactive to many nucleophiles. Esters are also carbonyl-containing species and have this feature; however, aldehydes and ketones are more reactive than esters.
The electrophilicity of the carbonyl carbon in esters is partially suppressed by resonance stabilization: the lone pair on the adjacent oxygen atom delocalizes into the carbonyl, reducing the positive character on the carbon.

This resonance effect makes esters less susceptible to nucleophilic attack than aldehydes or ketones.
This effect is even more pronounced in amides, where the nitrogen donates its lone pair more effectively, making amides the least reactive among the carboxylic acid derivatives.
Once again, the preparation and reactions of esters are covered in Organic Chemistry 2, and we only focus on recognizing and understanding their structure in Organic Chemistry 1 classes.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- How to Determine the Number of Lone Pairs
- Bonding Patterns in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis, and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
