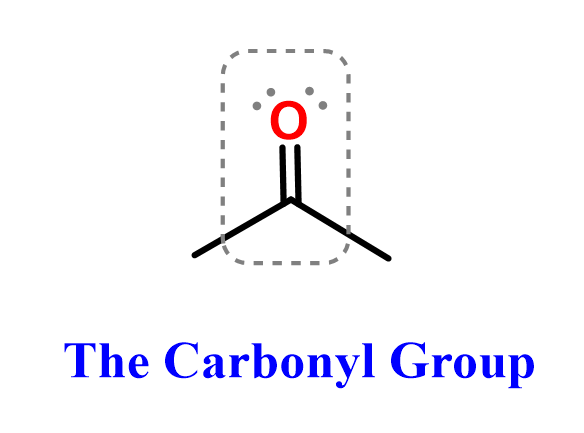
The ketone functional group is another common and reactive carbonyl-containing group in organic chemistry. Like aldehydes, ketones play a central role in nucleophilic addition reactions, oxidation-reduction processes, and are widely found in both biological molecules and industrial compounds.
Since ketones are carbonyl compounds, the C=O bond remains the central feature.
Structure of Ketones
In ketones, the carbonyl carbon is bonded to two carbon atoms (alkyl, aryl, or vinyl groups), unlike aldehydes, which always have at least one hydrogen attached to the carbonyl. The general structure is:
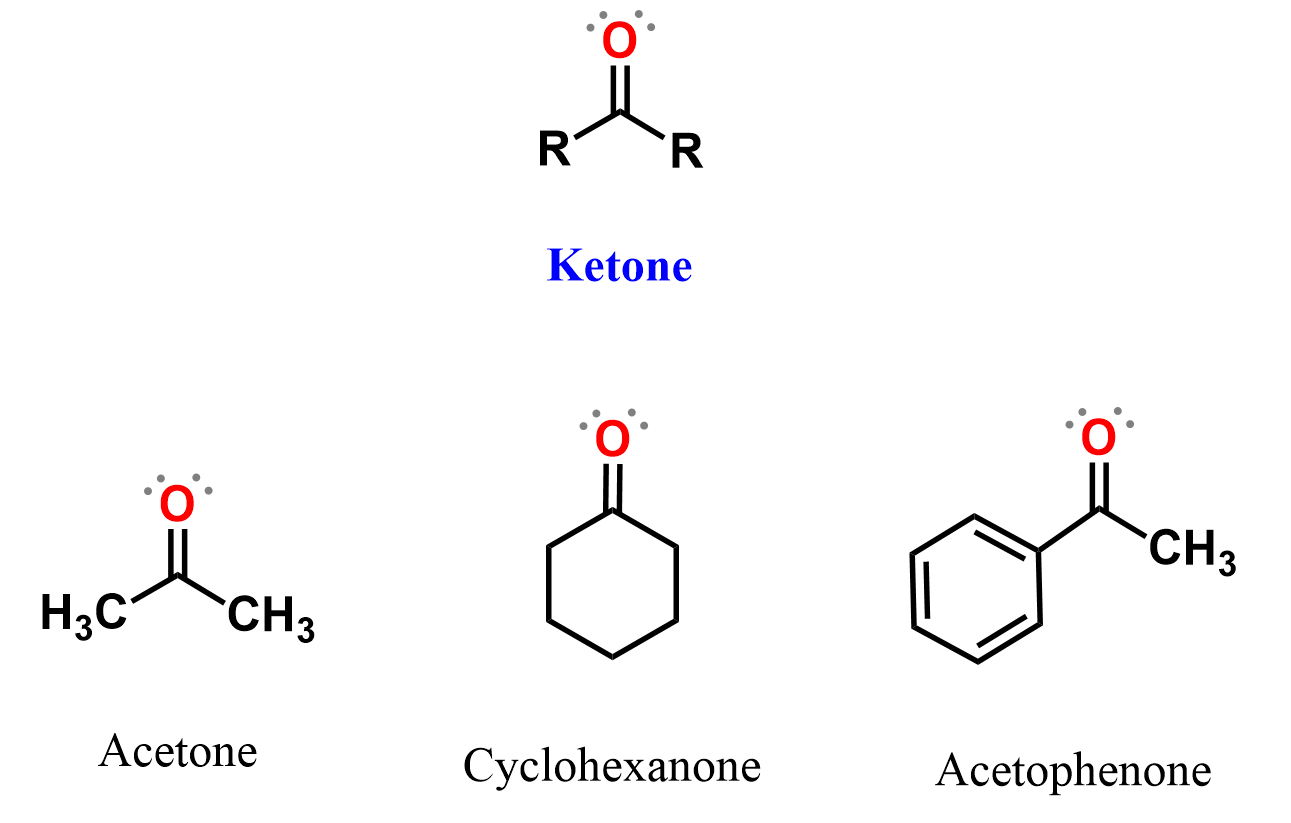
- R and R’ can be the same or different carbon groups.
- There is no hydrogen directly attached to the carbonyl carbon in ketones.
Nomenclature of Ketones
Ketones have lower priority than aldehydes. However, if it happens to be that the ketone is the highest priority in the molecule, then the suffix changes to “one”.
So, to name a ketone, we need to choose the parent chain such that it is the longest carbon chain that contains the C=O group:

The locant indicating the position of the carbonyl group can be placed before the parent or before the suffix “one.” Both names are acceptable according to the IUPAC recommendations. I.e., hexan-2-one or 2-hexanone would be suitable names.
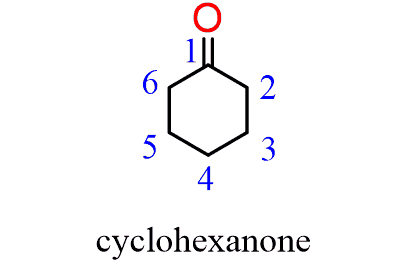
When naming a cyclic ketone, start numbering the ring beginning with the carbon connected to the C=O group. This rule always puts the =O group at C1, therefore, the “1” is usually omitted from the name:
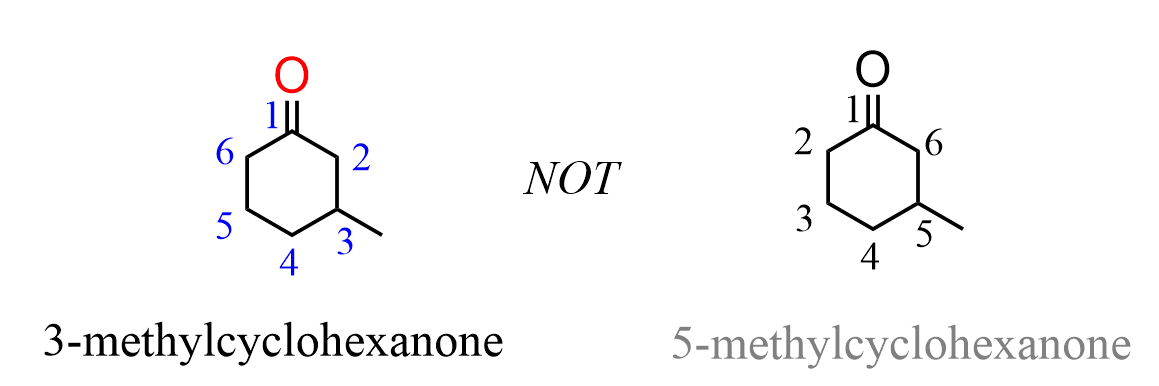
When other groups are present on the ring, it is numbered clockwise or counterclockwise depending on which direction gives the next substituent the lower number:
We have a separate post dedicated to the nomenclature of aldehydes and ketones, so check it out for more details, examples, and practice problems.
Geometry and Hybridization in Ketones
The carbonyl carbon in ketones is sp² hybridized, forming a trigonal planar geometry with bond angles of ~120°.
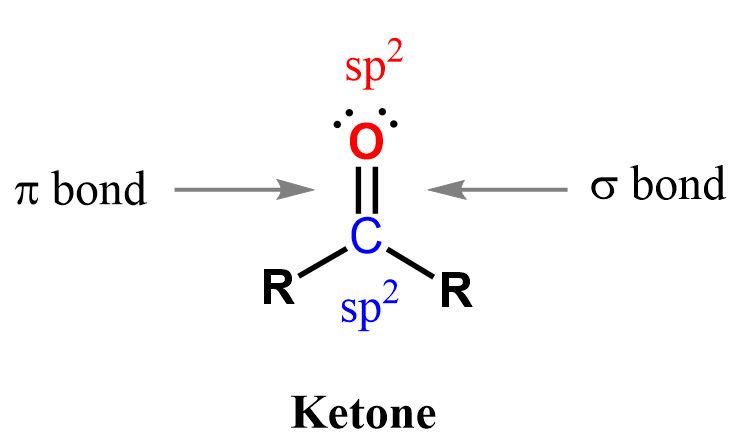
The C=O bond is polar, with a partial positive charge on carbon and a partial negative charge on oxygen. This polarity makes ketones reactive toward nucleophiles, mainly by addition-elimination mechanisms similar to aldehydes.
This is an Organic 2 topic, so you don’t need to worry about it if you are learning the functional groups.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- How to Determine the Number of Lone Pairs
- Bonding Patterns in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis, and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
