You have mastered the concept of resonance structures, can draw valid resonance transformations, and now all you hear is “that is a significant resonance structure” or “that is not a significant resonance structure.”
So, what are significant and non-significant resonance structures?
In essence, a non-significant resonance structure just means it is not a resonance structure relevant to the species you are considering. To make it even less confusing, for now, read it as a “wrong resonance structure.” It is technically not wrong, but it has no meaningful contribution to the actual structure of the molecule, which we call a resonance hybrid.
For example, let’s say you are given the structure of a ketone, and need to draw a resonance structure for it:
From all you have learned about resonance structures, you are likely (and hopefully) going to move the electrons of the pi bond to the oxygen and place the correct number of lone pairs and formal charges on the corresponding atoms:
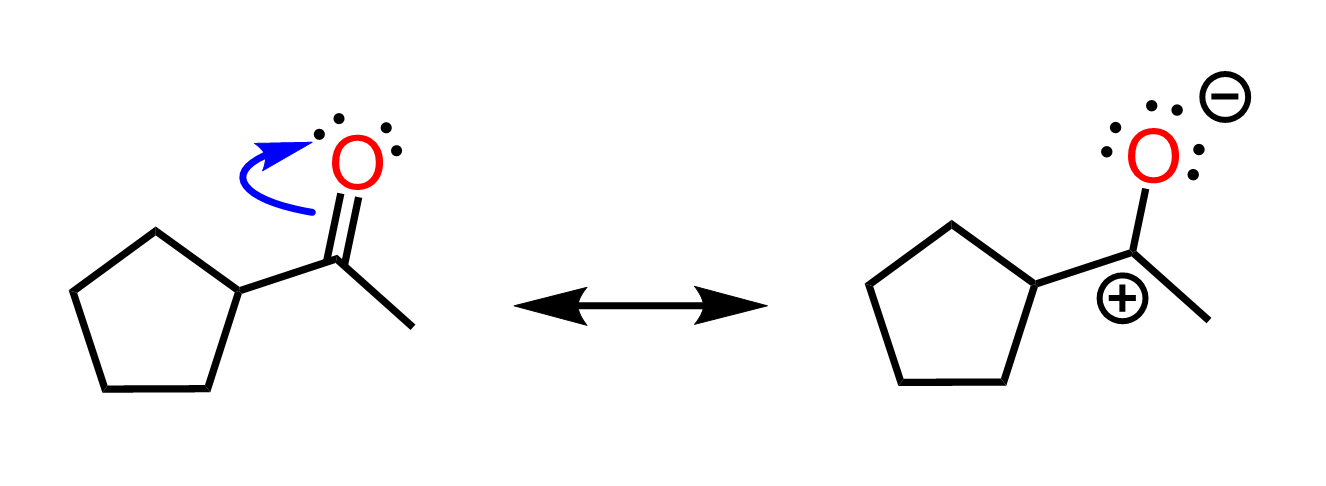
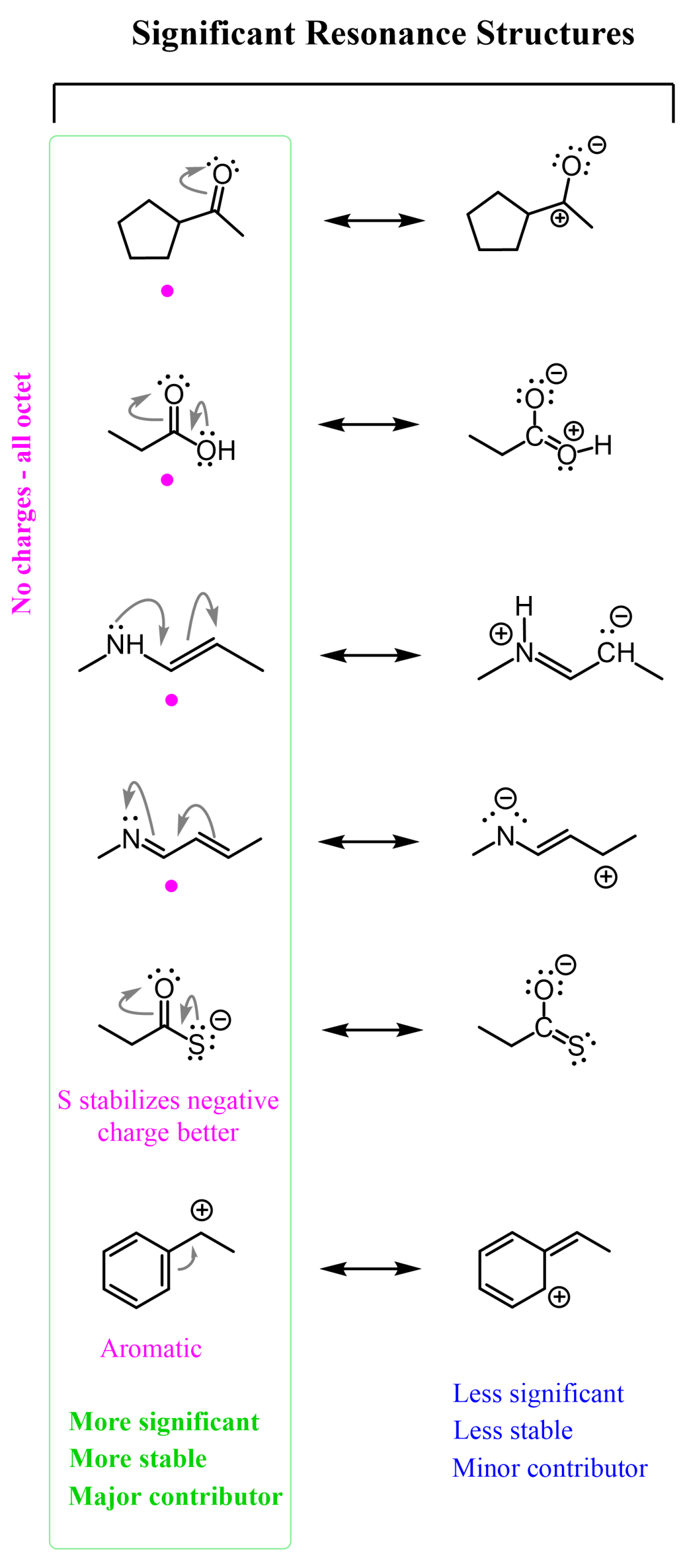
This is another resonance form of the given ketone. It is less stable (less significant), but it is a significant resonance form. The more significant one is the original structure, where all the atoms have octets and there are no formal charges. We also call this the most significant resonance structure, or the most stable, or the major contributor to the resonance hybrid. Here are some more examples of significant resonance structures. Among them, we have the more/most significant and less significant contributors to the resonance hybrid:
Now, why can’t we move the electrons of the pi bond in the other direction and place partial positive and negative charges on the oxygen and carbon, respectively?
We could, and these are what we call non-significant resonance structures.
Non-Significant Resonance Structures
Let’s go against what we learned in general chemistry and take electrons from a more electronegative oxygen and give them to a less electronegative carbon:
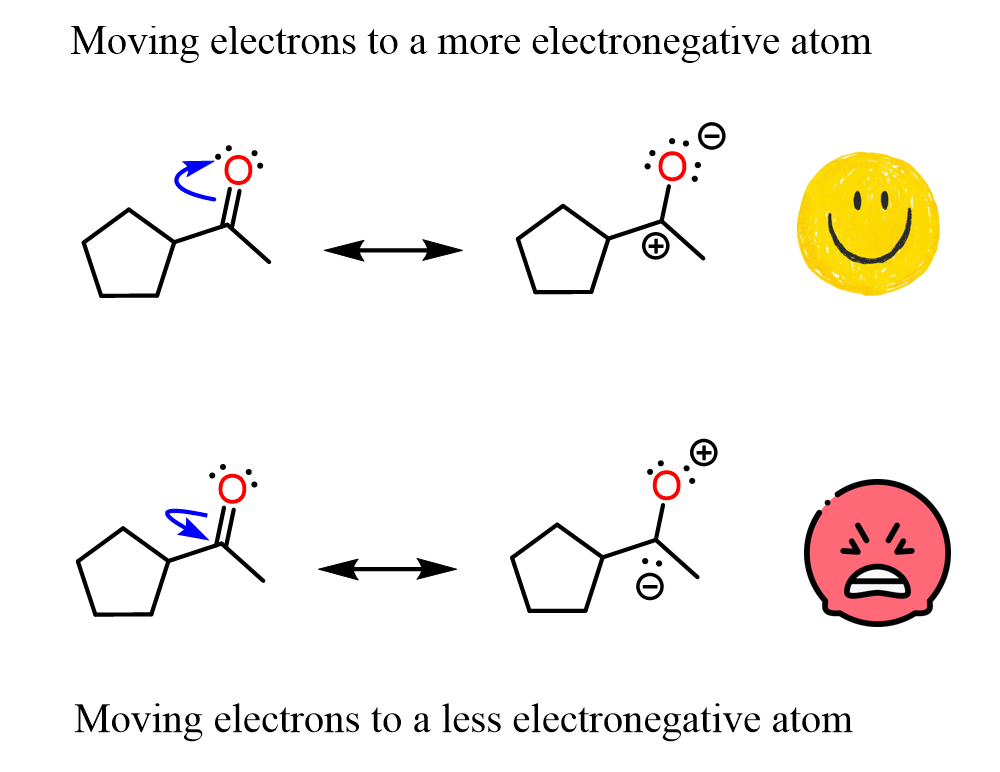
Hopefully, it looks ugly or at least counterintuitive to you, because we don’t move electrons from a more electronegative atom (O) to a less electronegative atom (C).
Structures with such an electron distribution are not significant contributors to the resonance hybrid. They are still resonance structures, but they have no meaningful message to tell us about the electron distribution in the molecule. Remember, resonance structures differ only in electron distribution – all the atoms have the same connectivity.
So, the question: why not call them wrong resonance structures or not call them resonance structures at all?
Talking about the example we are discussing, we can see that it is technically not wrong because the oxygen and carbon have the orbital capacity to accommodate the given number of electrons. It would be wrong or impossible if we, for example, pushed one of the lone pairs on the oxygen and made a triple bond with the carbon. That would certainly be impossible, as the carbon does not have those orbitals to accommodate 5 bonds. Here is an illustration of significant and non-significant resonance forms:
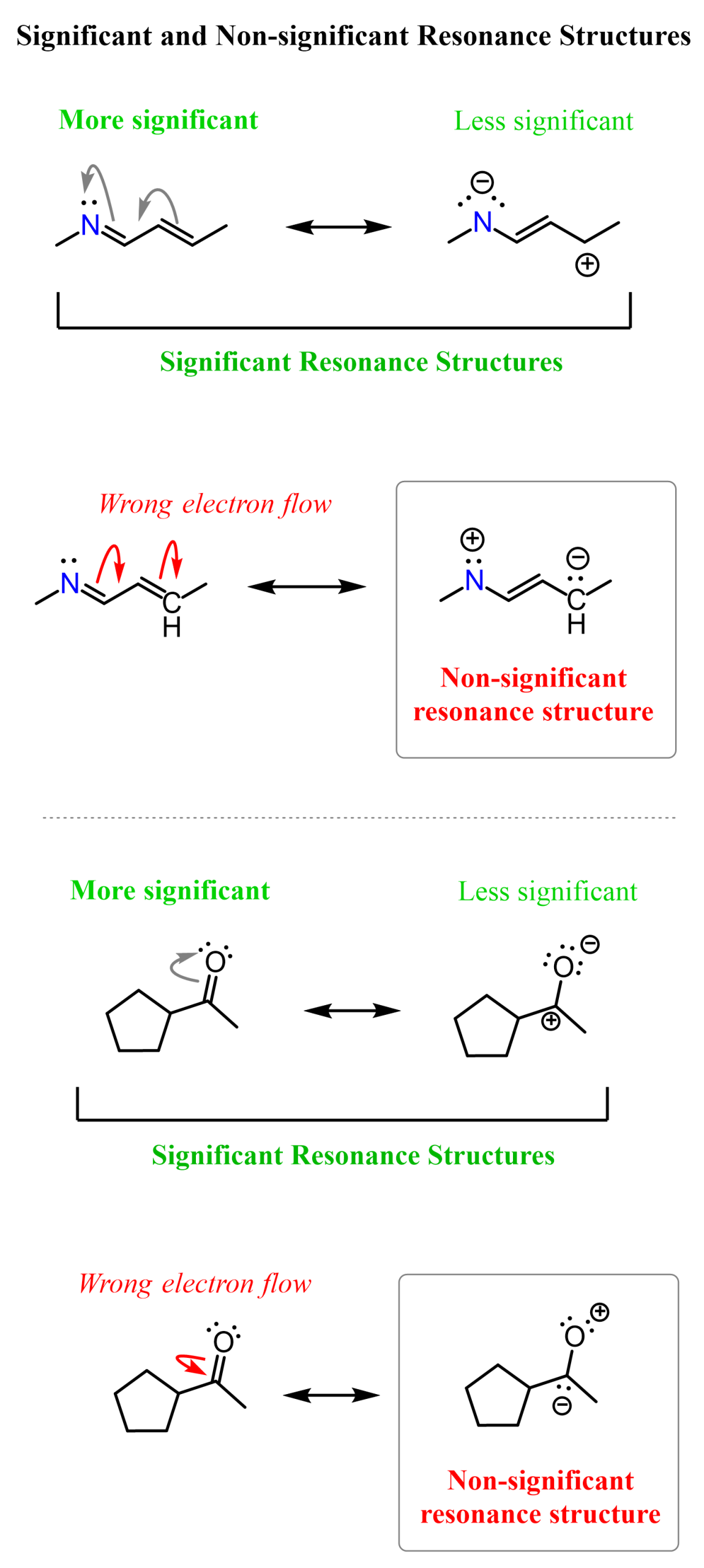
To summarize, once you know what resonance structures are and know the rules for drawing them, you will say that significant resonance structures are those that make sense, and those that don’t are non-significant, although they do not exceed octets on second-row elements.
To avoid drawing non-significant resonance structures, simply do not push electrons from a more electronegative element to a less electronegative element. If charges are present and such electron flow results in a neutral molecule, then yes, it makes sense, but not if you’re starting from a neutral molecule.
In both examples above, it makes sense to push the electrons from negatively charged nitrogen and oxygen to the positively charged carbon (reverse direction), as that creates a perfectly neutral species.
The terms most significant, most stable, and major contributor are synonyms – they simply mean the most stable resonance structures.



