This comprehensive set of practice problems on Stereochemistry includes questions on determining R and S configuration, R and S configuration in Fischer and Newman projections, and determining the relationship between molecules such as enantiomers, diastereomers, and constitutional isomers. There are also questions on optical activity, such as determining the optical rotation, the enantiomeric excess, the percentage of each enantiomer, and more. Towards the end, we also practice meso compounds, Fischer projections, converting between Fischer and Newman projections and vice versa.
This is quite a lot, but we have even more. As a CS member, you will also get access to this over 100-question multiple-choice stereochemistry quiz and all the quizzes as well.
Stereochemistry Practice Problems Quiz
This video is a fragment the quiz and explains the rules for drawing Fischer projections in the context of determining the relationship between given compounds. The quiz comes with a 3-hour video solution.






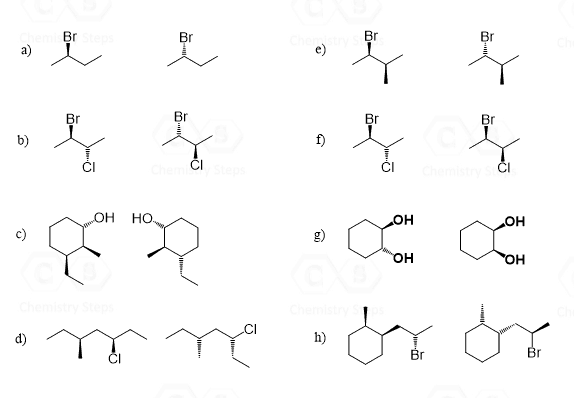
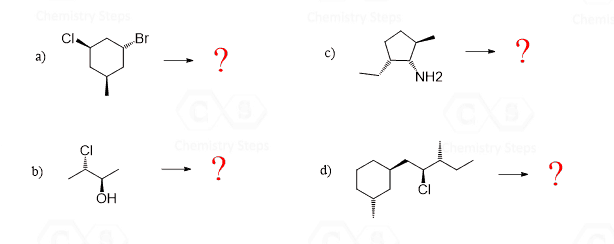
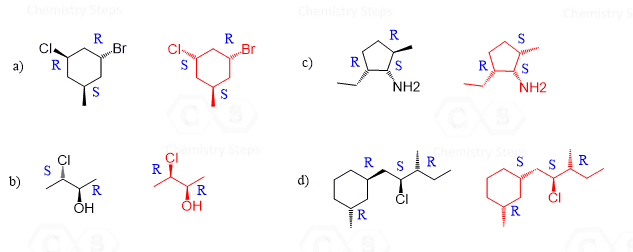


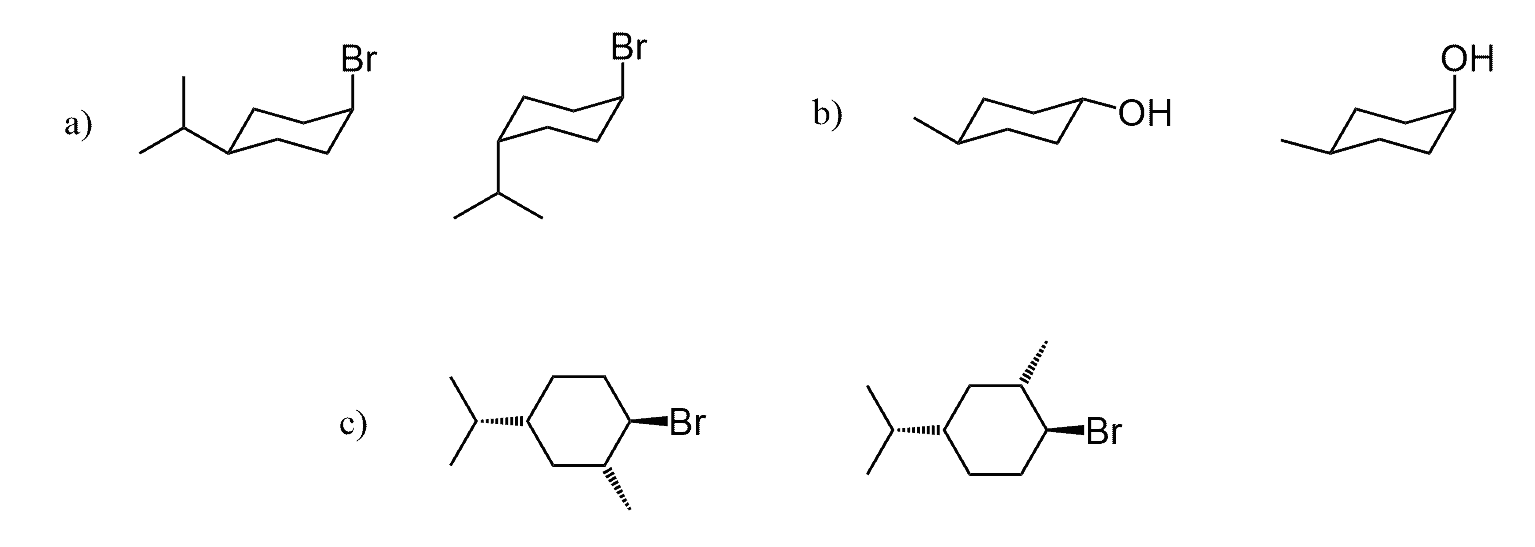

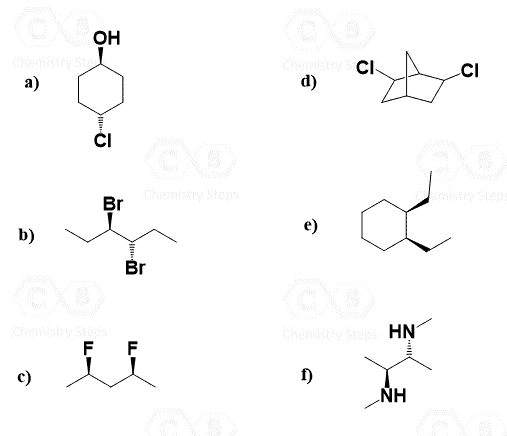



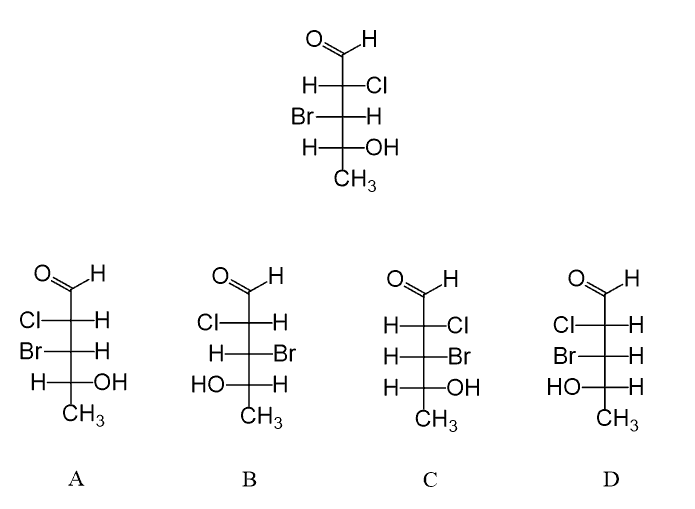
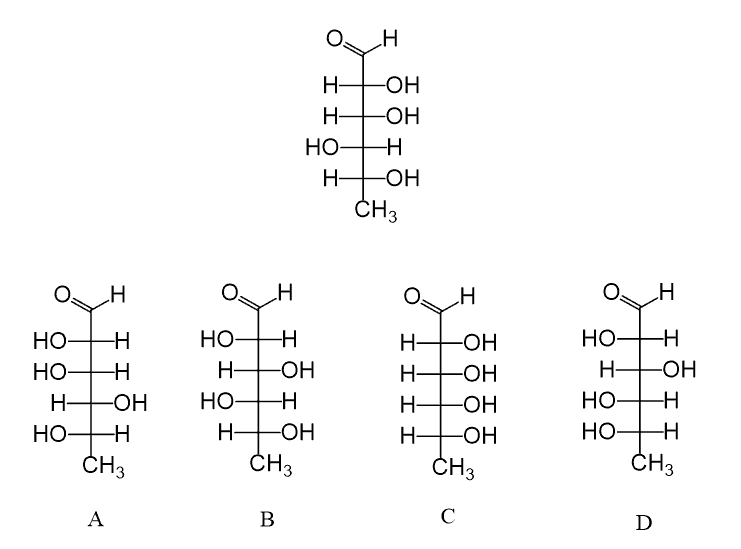
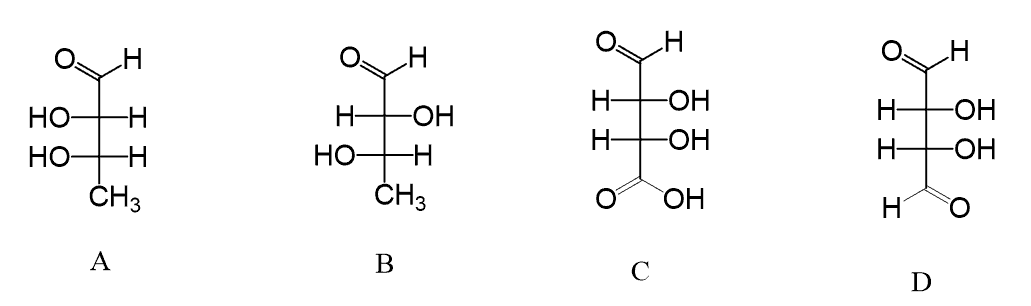
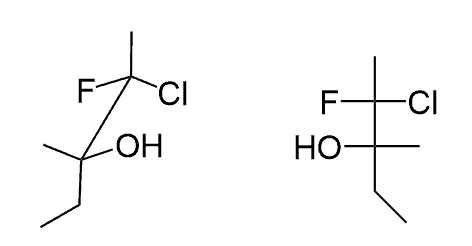
Hi, in question 8- b why Br is pointing towards but in f Cl and F are pointing away? both b and f they have a identical bond?
and second question if we want to flip a molcule in a bond line dash becomes a wedge? or always remain dash a dash after fliping?
Thank you in advance
Hi there,
For the first question, I’ve already responded to it under another post, but in case you missed it, I’m quoting my answer here again. Please don’t hesitate to let me know if you’d like further clarification.
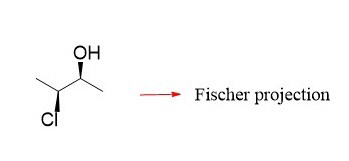
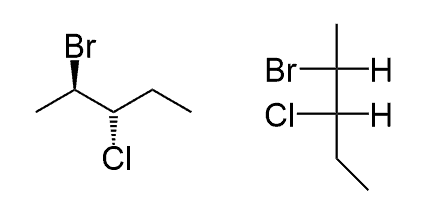
“I am assuming you are referring to the fact that “towards us” in bond-line notation indicates a wedge, but here it is a dash. The difference in bond-line and Fischer notations is the view angle. In Fischer, we are looking from the top, and both wedge and dash are pointing towards us, whereas in bond-line, we are looking from the side, and it is the wedge group that is pointing towards us.
See how the wedge Br and dash H are pointing towards us when converting to a Fischer projection in this example:
Check also this article for more examples on converting Bond-line to Fischer projection and vice versa.”
Question 2: Whether you need to flip the wedges and dashes depends on what you are trying to represent and from which angle you are viewing the molecule. There is no single rule that always requires changing or keeping them.
• Same molecule, different orientation: if you rotate the molecule 180° in the plane of the paper, the wedges and dashes stay as they are, but the atom/groups that were pointing up will now point down, and vice versa.
• If you flip the molecule outside of the paper, then yes, all the wedges and dashes will be switched.
• The Enantiomer: if you want to draw the mirror image, keep the zigzag backbone the same and flip all the wedges and dashes. See this image from the post “Enantiomers vs Diastereomers.”:
I recommend using a molecular model and physically rotating it to see how the bond projections change. Wedge and dash notation is relative because it depends on your viewing angle. What ultimately matters is the absolute configuration (R/S) at each chiral center. It may not be practical to determine the R and S every single time, and there is still a chance of making a mistake in doing that, so having a model to improve 3D visualization would be very beneficial.
Thank you very much. your explanation was very helpful and thorough.
You are most welcome. Feel free to ask your questions!