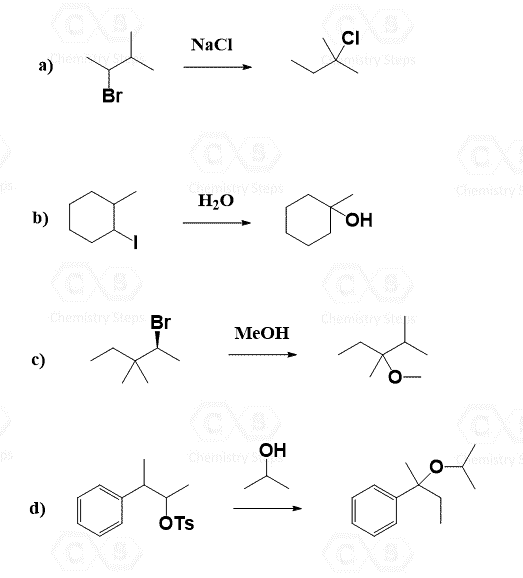
Now that you have learned about the SN1 and SN2 mechanisms, try to draw a reasonable mechanism for the following substitution reaction:
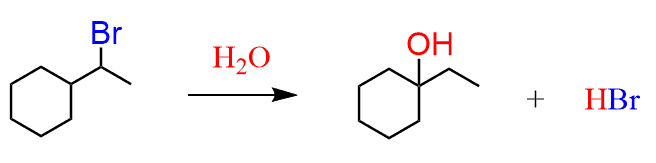
Wait, this doesn’t make sense, right? The nucleophile suddenly appears where there was no leaving group…
Ok, so that is the point – whenever you see something like this where the positions of the nucleophile and the leaving group do not match, think about a rearrangement reaction.
What is a rearrangement reaction, and how does it work?
A rearrangement is a change of connectivity in the molecule as a result of a Hydride or Methyl shift. To explain why and how this happens, you need to recall the stability of carbocations:
More substituted carbocations are more stable because of the electron-donating effect of alkyl groups and the hyperconjugation.

Hyperconjugation is the charge-stabilization by pushing some electron density of the adjacent σ bond to the empty p orbital of the carbocation:

Let’s emphasize this once again: for a rearrangement to occur, the formation of a carbocation is necessary. Carbocations are only formed in unimolecular (SN1 and E1) reactions since the SN2 and E2 reactions are concerted mechanisms where everything happens at the same time.
So, at his point, we know that this reaction must have been an SN1 mechanism since there is no double bond formed (no E1). Now, let’s draw the first step of the SN1 mechanism. Remember, it is the loss of the leaving group, which is the rate-determining step:

The resulting carbocation is secondary, and this means it has a chance of becoming more stable, and it will do it through a rearrangement. The purple hydrogen jumps with the bonding electrons (that is why it is called a hydride) to the next carbon with a positive charge. The result of this is the formation of a more stable 3o carbocation:
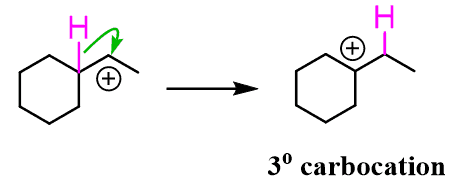
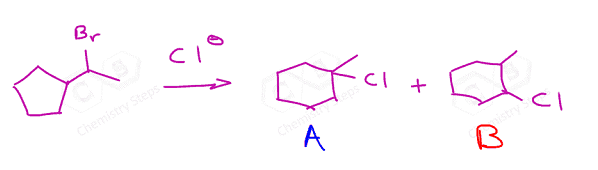
In general, a rearrangement happens only if the resulting carbocation is more stable. Most often, this means a more substituted carbocation, but resonance-stabilized carbocations can also be the driving force:

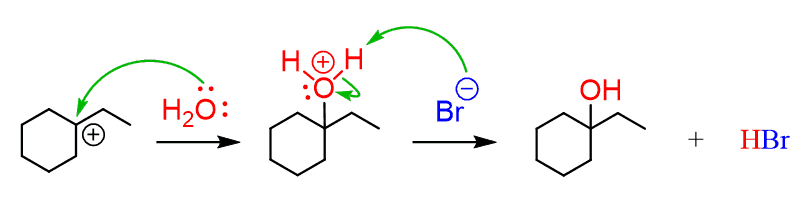
Going back to our reaction: after this, the nucleophilic attack and deprotonation are followed, which leads to the final product:
1,2 Hydride and 1,2 Methyl Shifts
Let’s put all these together to summarize what happened in this rearrangement reaction:

This specific reaction of a rearrangement was a 1,2-hydride shift. The numbers are to emphasize that this shift can only happen from adjacent carbons. You cannot move the hydride ion 5 bonds away just because it gives a more stable carbocation.
The other type of rearrangement is the 1,2-methyl shift. Here, instead of the hydride ion, we have a methyl ion with the electrons (sometimes referred to as methide ion) jumping to the next carbon to create a more stable carbocation:

What about the stereochemistry in rearrangement reactions?
The short answer here is that there is no stereochemical control since it is part of the unimolecular SN1 and E1 reactions. Remember, the moment you form a carbocation from the chiral center, the stereochemistry is gone; you are only going to get a racemization of that center.
Now, let’s, for example, pick the R configuration of the alkyl chloride above and see what happens to the stereogenic center:
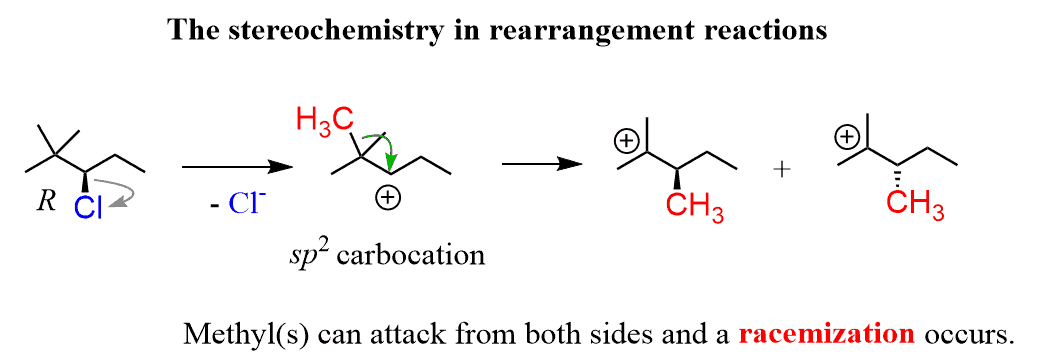
The stereogenic center is lost right when the Cl leaves, and the second one where the methyl shifts forms in equal amounts.
Even if the carbon where the alcohol attacks became a stereogenic center, it would still be racemized.
Ring Expansion Rearrangements – Brief Overview
Ring expansion rearrangements occur when a carbocation formed during a reaction rearranges to relieve ring strain or form a more stable carbocation. This is especially common in small ring systems like cyclobutanes, where expanding the ring reduces angle strain and leads to a more favorable structure.
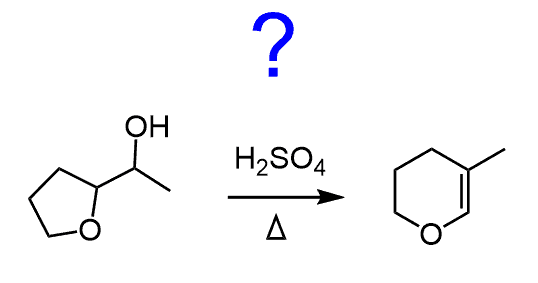
For example, the hydrolysis of the following alkyl halide gives an alcohol where the cyclobutane ring is converted into a cyclopentane. This happens through a carbocation rearrangement that expands the ring by one carbon before the nucleophilic attack of water or other weak nucleophiles.
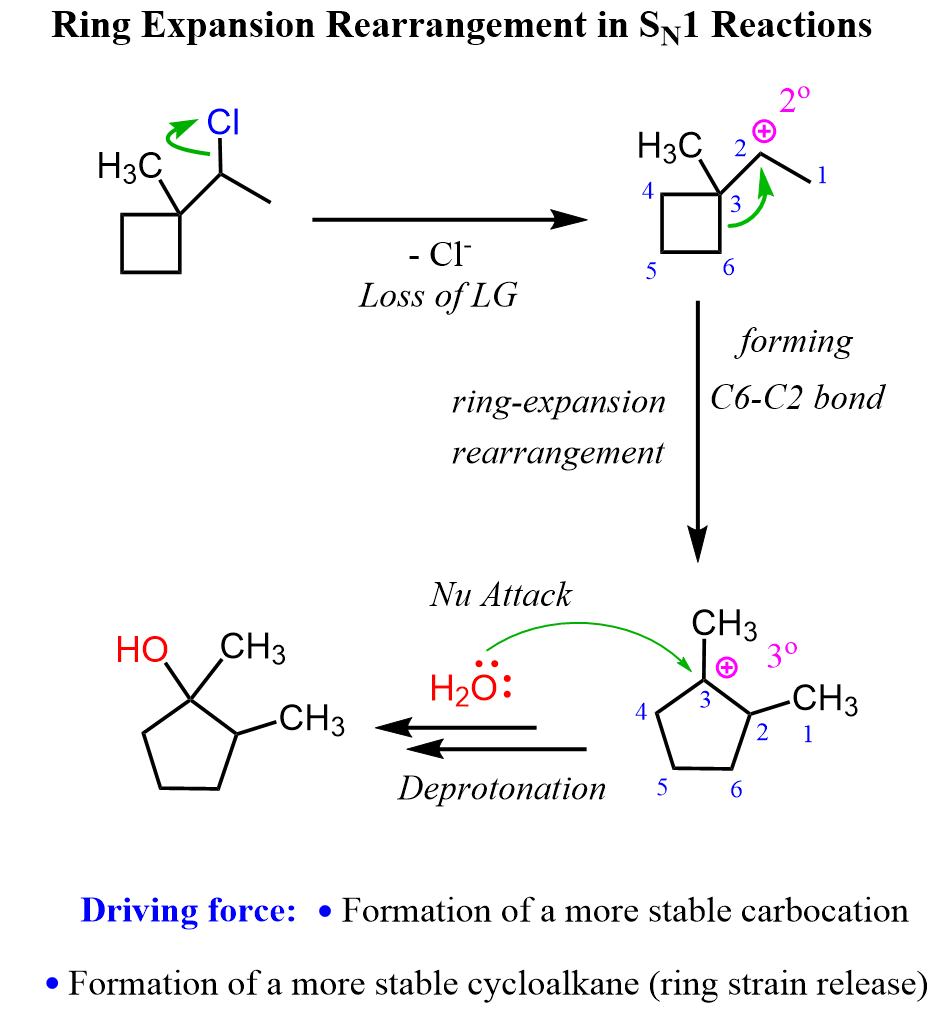
We have a separate post on ring expansion rearrangements, so make sure to check that as well for more detailed examples and step-by-step mechanisms!
Pinacol Rearrangement
An important type of rearrangement takes place when 1,2-diols (vicinal diols) are treated with acid. The diol here is converted into a ketone, and this is known as the pinacol rearrangement.

The mechanism and some other details of the pinacol rearrangement are covered in a separate post, which you can find here.