Determine the Relationship of molecules
Enantiomers, Diastereomers, Constitutional isomers, Same or no relationship? A question that covers so much for you to learn, yet a very important one as it lays the basis of understanding organic chemistry.
Let’s put this chart flow and start from the concept of isomerism in general:

Iso- means same, so, in order for any two molecules to be isomers, they must have the same chemical formula. But, of course, not any structures with the same chemical formula are isomers, as they may just be two different drawings of the same compound. Therefore, isomers are different compounds with the same chemical formula.

Notice that the atoms are connected differently. And this is the simplest class of isomers which we call Constitutional (Structural) Isomers.
So, we are right here:

Stereoisomers
The next class of isomers the Stereoisomers. Two examples:

Notice the difference with constitutional isomers – in stereoisomers, the atoms are connected the same, however, some of them have a different arrangement. In the first pair, the Br is on position 2, but it is pointing towards you and away from on the second molecule.
For the second, pair, both the Br and Cl are on the same position, however, they point differently and that is what makes the two molecules stereoisomers. We are here:
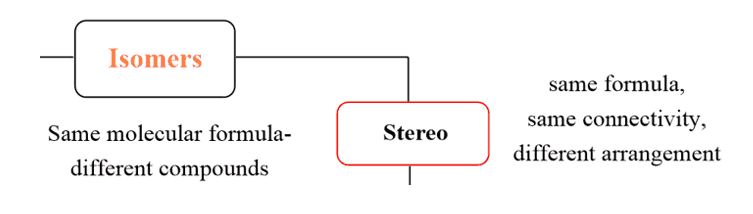
Enantiomers
This is a good time to remember about enantiomers. Enantiomers are two molecules that are nonsuperimposable mirror images:

And this is what we had for our pairs of stereoisomers, they were nonsuperimposable mirror images – enantiomers. The lesson is that enantiomers are stereoisomers.
Remember also that all the chirality centers are inverted in enantiomers, every R is changed to an S and every S is changed onto an R.

Diastereomers
Now, not all the stereoisomers are enantiomers. Stereoisomers are also divided into two main groups.
For example, compare these two molecules. What is their relationship?

We have two chiral centers, and only one is changed, while the other one is the same. So, these molecules are not mirror images (in mirror images, everything is reflected, therefore changed). And this is what we call Diastereomers: Diastereomers are stereoisomers that are not mirror images of each other.
So, we already completed the chart:

Diastereomers have at least one chiral center where the R, S configuration is the same and one where it is inverted. In other, words, in diastereomers, some of the chiral centers are the same, and some are different:
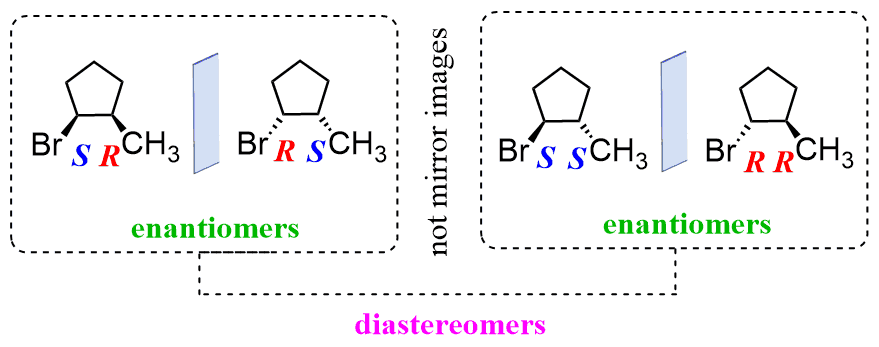
Let’s now go into some tricks.
Meso compounds
These are molecules with stereogenic (chiral) centers that contain a plane of symmetry. Importantly, this plane of symmetry makes them achiral despite the presence of the chiral centers:

So, if you are given two molecules, where every chiral center is inverted, do not hurry to classify them as enantiomers. Check for a symmetry plane.
- If the molecule contains a symmetry plane, it cannot be chiral, therefore it cannot have an enantiomer.

Cis and Trans Isomers
You may also need to classify two molecules with a cis/trans double bond or a ring system.

Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers. So, cis and trans isomers are diastereomers.
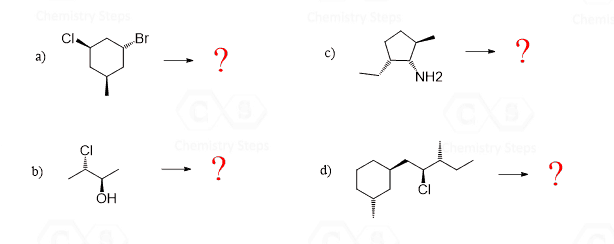
In the following practice problems, I put questions with different difficulty levels. For some, you will be able to determine the relationship just by visual assessment, some will require determining the R and S configuration, there are also the ones where you compare different representations such as Newman vs Fischer, or a bond-line vs sawhorse.


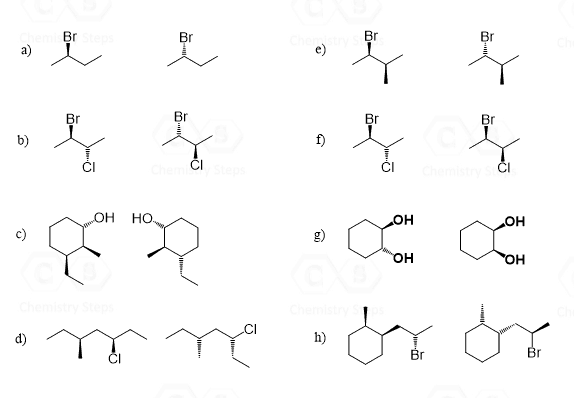
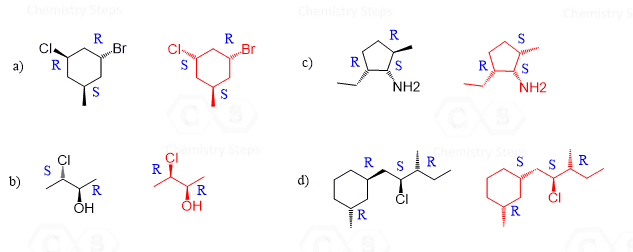



Why is it that they are diastereomers in question g, exercise number 3? Aren’t diastereomers supposed to have two chirality centers; one being the same and one inverted? I determined the absolute configuration of both chirality centers and they are identical (S, S). Help please.
R-Manez,
Yes, diastereomers generally have two or more chiral centers but this is not a requirement. In fact, diastereomers do not have two have chiral centers. For example, Cis- and trans-isomers are diastereomers since they are stereoisomers that are not mirror images of one another. In this example as well, the two molecules are Cis- and trans-isomers and the absolute configuration of the chiral centers wouldn’t make any difference even if it was inverted in both molecules.
they are distereomers because one is trans and other is cis
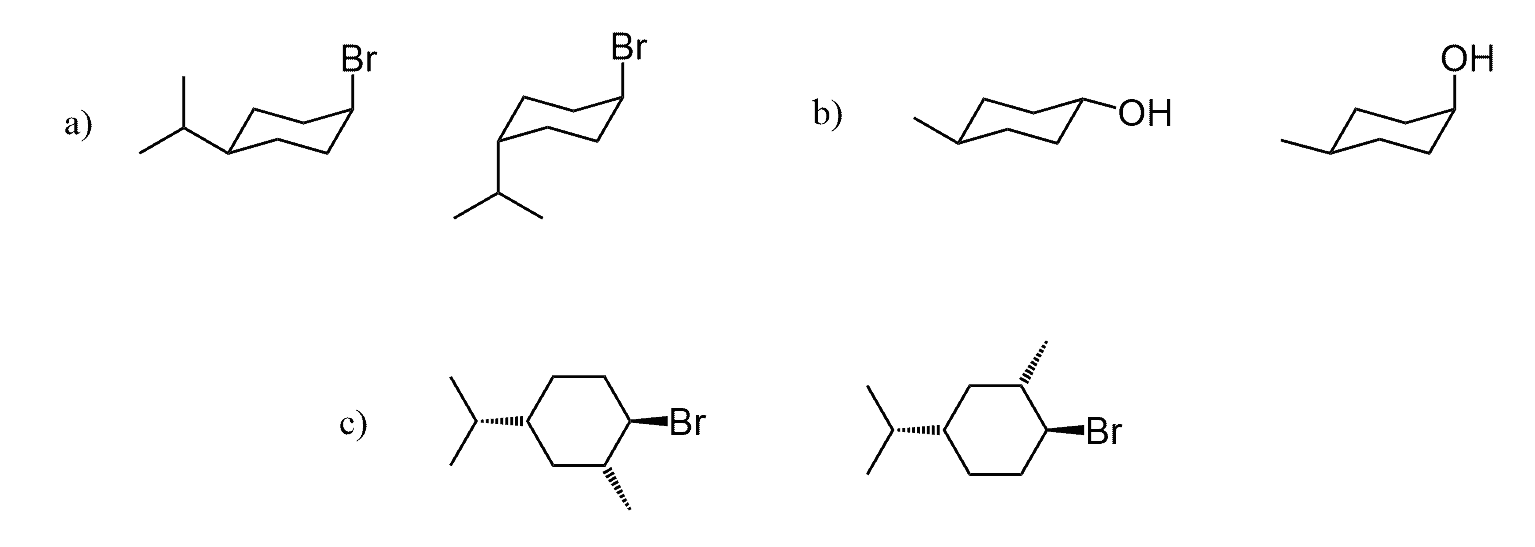
I get why “a” and “b” in question 5 are diastereomers but what makes “c” enantiomers? Aren’t they the same once we do a ring flip?
Hi Sean,
It may be helpful to redraw them next to each other and add a mirror plane between them. This will reveal that they are non-superimposable mirror images with identical connectivity and therefore, are enantiomers:
Another way to look at it, is by determining the absolute configurations of chiral centers. Each chiral center must be of opposite configuration if the molecules are enantiomers:
For the ring flip: It is always a good idea to number all the carbons in the ring and mark the groups as “up” and “down” even though this a relative assignment as they can be up or down depending what direction you are looking at them. As it is drawn, we see the wedges pointing towards us, so we mark them as “Up” and dashes pointing away from us, so we mark them as “Down”.
There is no difference in choosing which chair conformation to start with as long as you keep all the chiral centers correct, or simply follow the “Up” and “Down” representation.
Once you have the chair with the correct configuration of chiral centers, you can do the ring flip.
This also confirms that they are enantiomers since both conformations have their corresponding non-superimposable mirror images. It is crucial to keep the direction of numbering the ring consistent-check the post about the ring-flip.
Woohoooo, this is too much )))
in question 3, why is c the same whereas g are diastereomers?
i am also confused how is the answer for f an enantiomer
Hello,
Sorry, did you have a chance to watch the video solution?
How can you figure out what type of isomer it is by assigning R and S configuration?
The R and S configuration can be used to distinguish molecules that have chiral centers. Recall that some diastereomers such as cis and trans isomers do not have chiral centers. If there is only one chiral center, the molecule can only have an enantiomer (there is always only one enantiomer regardless of how many chiral centers the molecule has). So first make sure there are chiral centers, then remember that in enantiomers, all the chiral centers are inverted, whereas in diastereomers, not all of them are. It can be that only one is, and all the others are the same, it can be that all are inverted but one, and any other combination except where all are inverted. This image should clarify it better:
The enantiomer of each molecule is drawn next to it. Notice again that all the chiral centers are inverted.
Hi. Question 6 (a) is enantiomers? Br is dash and in Fischer we said it should be point toward us. dont we need to rotate it? and in this case the answer will be same identical?
Hi there,
Did you have a chance to watch the video solution? It is hard to visualize what exactly is confusing you. I am assuming you are referring to the fact that “towards us” in bond-line notation indicates a wedge, but here it is a dash. The difference in bond-line and Fischer notations is the view angle. In Fischer, we are looking from the top, and both wedge and dash are pointing towards us, whereas in bond-line, we are looking from the side, and it is the wedge group that is pointing towards us.
See how the wedge Br and dash H are pointing towards us when converting to a Fischer projection in this example:
Check also this article for more examples on converting Bond-line to Fischer projection and vice versa.