When we learned about enantiomers, we mentioned that they have identical physical properties – melting point, boiling point, and solubility. So, one question you may wonder about is how we distinguish enantiomers if they all seem to be identical. Yes, we know that they are not identical, but rather they are nonsuperimposable mirror images. The problem, however, is that we cannot easily observe molecules and therefore, the idea about nonsuperimposable mirror images is great on paper but not as practical when working with actual molecules.
Luckily, one of the few features that sets them apart is their interaction with plane-polarized light. So, first, what is the plane-polarized light?
Plane-polarized light
Ordinary (unpolarized) light consists of electromagnetic and magnetic fields propagating through space. These waves oscillate in all planes perpendicular to the direction in which the light travels.
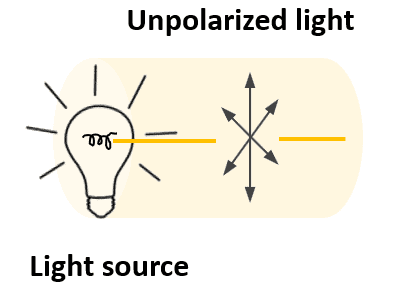
If we place a device called a polarizer next to the light source, it acts as a filter which only lets through light waves that are oscillating in a single plane.
This light is now converted into what is called a plane-polarized light (or simply polarized light).

The plane-polarized light is still seen as the normal light since we cannot see these changes in with a naked eye. For example, if it is a yellow light, it is still going look just the same as before it was polarized.
So, what is the advantage of creating this light with a unidirectional electromagnetic field?
An interesting observation was first made in 1815 by French scientist Jean Baptiste Biot who noticed that the plane of a polarized light rotates when it passes through the solutions of some carbohydrates (sugars). Based on this property, organic compounds started to be classified as optically active or optically inactive. More specifically, the compounds that rotate the plane of a polarized light are said to be optically active. And the ones that do not rotate the plane pf a polarized light are optically inactive.

It is important to know that optically active compounds are chiral and optically inactive compounds are achiral.
The instrument that is used to put all these parts together and allow for measuring the extent and the direction to which the plane rotates is called a polarimeter. The light source in a polarimeter is usually a sodium lamp, which emits a light at 589 nm fixed wavelength known as the D line of sodium.
If we were to sketch a polarimeter using the image above, it would only be missing a detector that measures the degree of the rotation. To achieve, this, another filter is placed right at where the light comes out of the tube. This filter also passes the light with only one oscillating plane and therefore, the light can only be detected if the filter is set at the same angle as the plane of the light.

This requirement for detecting the light coming out of the sample, allows to measure the degree of rotation caused by the interaction of the light with the chiral sample.
Observed Rotation
In addition to the angle, the direction of this rotation also needs to be recorded as it is a unique characteristic of the given enantiomer.
If the rotation is clockwise, the compound is called dextrorotatory, and the rotation is labeled d or (+). On the other hand, if the plane of the light rotates counterclockwise, we have a levorotatory compound, and the rotation is labeled l or (–).
This notation is called the observed rotation (ɑ) of the sample.
In the example on the diagram, the plane of the light has rotated in a clockwise direction (hopefully it is seen that way) at 90o. Therefore, it is a dextrorotatory (d) or (+) compound and the observed notation would include both the degree and the sign of the rotation; ɑ = +90o.
Optical Activity and Enantiomers
This is a very important information you want to keep in mind as it will be needed in solving problems related to the optical activity, chirality, observed rotation, specific rotation, and enantiomeric excess.
Remember that enantiomers rotate the plane of the polarized light to an equal extent but in the opposite direction. For example, if a compound with a given concentration measured under identical conditions has an observed rotation of +20o, then its enantiomer will rotate the plane of a polarize light to by -20o.
If the sample is a mixture of two enantiomers, then the rotation will be directed by the one that is present is a larger quantity. More specifically, the excess amount of this enantiomer will rotate the plane of the polarized light. We will cover this is more detail in the post dedicated o the enantiomeric excess.
Notice also that we mentioned about identical conditions for carrying out the experiment since the optical rotation depends on the concentration of the compound, the length of the sample tube and the temperature. Therefore, to keep the data universal and comparable regardless of the concentration, the observed rotation is converted to what is called a specific rotation. We will cover this in the next article as it will also involve a little portion of math.
R and S, d and l and (+) and (-)
Here, you will need to remember that there is NO relationship between the R and S configuration and the d (+) and l (–) notations. The R and S notation is used for labeling the absolute configuration of chirality centers while the d (+) and l (–) notations indicate the direction to which the sample rotate the plane of a polarize light.
For example, the S enantiomers of glyceraldehyde and lactic acid are levorotatory (–) and dextrorotatory (+) respectively.

Are Polarized Glasses Related to Polarized Light?
A practical application that can be appreciated by people not takin organic chemistry, is the improvement achieved by polarized glasses.
The sunlight is unpolarized until it is, for example, reflected from the surface of the road. The reflected waves are polarized mainly in the horizontal plane and if we use sunglasses with vertically polarized lenses, it will get rid of the reflected light and enhance our vision. This is how polarized glasses can greatly improve our vision, especially when driving for a long trip on a sunny highway.

Notice on the picture that if we align two pairs of polarized sunglasses at 90° the plane-polarized light is blocked, and we couldn’t see the jellybeans anymore.
Check out this 108-question, Multiple-Choice Quiz with a 3-hour Video Solution covering the most important concepts you need to know in Stereochemistry:
Stereochemistry Practice Problems Quiz
Check Also
- How to Determine the R and S Configuration
- The R and S Configuration Practice Problems
- What is Nonsuperimposable in Organic Chemistry
- Chirality and Enantiomers
- Diastereomers-Introduction and Practice Problems
- Enantiomers vs Diastereomers
- Cis and Trans Stereoisomerism in Alkenes
- E and Z Alkene Configuration with Practice Problems
- Enantiomers vs Diastereomers
- Enantiomers, Diastereomers, the Same or Constitutional Isomers with Practice Problems
- Configurational Isomers
- Specific Rotation
- Racemic Mixtures
- Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
- R and S Configuration of Allenes
- Converting Bond-Line, Newman Projection, and Fischer Projections
- Resolution of Enantiomers: Separate Enantiomers by Converting to Diastereomers
- Stereochemistry Practice Problems
- Stereochemistry Practice Quiz


Best