You might have heard the term “Nonsuperimposable Mirror Images” during the lecture but did not quite understand what it was and how it was related to enantiomers, diastereomers, or other types of correlations between organic molecules.
So, what does it mean to be superimposable or nonsuperimposable to another molecule? Superimposable means two objects, whether molecules or not, can be positioned indistinguishable from each other even though, as shown, they may not look the same. If molecules are superimposable, we can essentially merge and make them as one:
There would be countless such examples because any object can be drawn/projected in different ways depending on the view angle, and all we need to superimpose them is to move them around or change the viewing angle.
As an analogy of regular objects and molecules, compare the two representants of a marker and butanol:

They are the same entity drawn differently, and therefore, can be superimposed by a 180o flip.
Mirror Images
Let’s now narrow it down from superimposable to superimposable mirror image. Place a mirror next to any object and compare the two images. Imagine the mirror image being a real object, and try to superimpose it with the original object.

If you can repeat the look/representation of the original image, then the mirror image is said to be superimposable to its mirror image. All the pairs above are superimposable, as we simply need to move them, and they will become indistinguishable. In other words, we can say that superimposable means identical – if two objects can be superimposed, then they are identical.
Nonsuperimposable Mirror Images
Some objects though, are not superimposable with their mirror images. The best example is our hands: they look similar and perhaps identical at times, but they are not – they are nonsuperimposable mirror images:
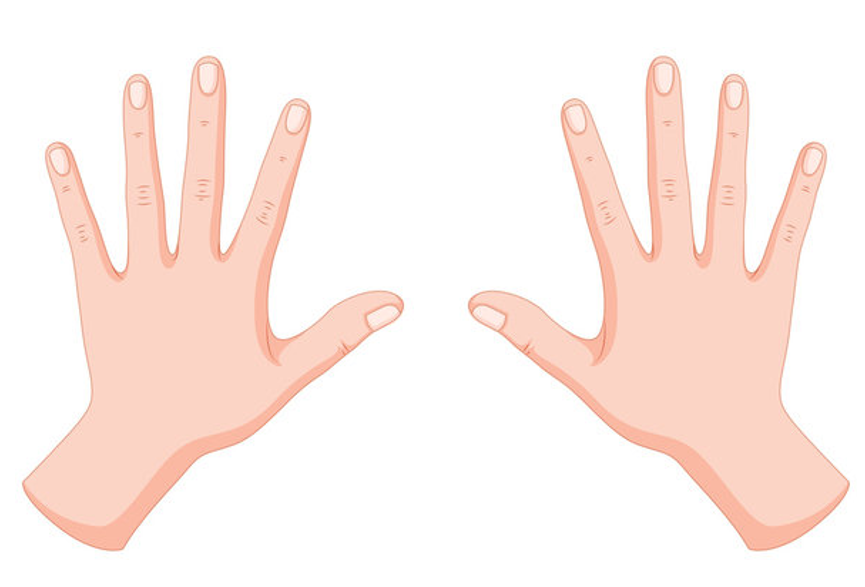
Another example would be glasses with different lenses. They look the same as their mirror image, but if we replace one of the lenses with a dark lens, the mirror image is now different – you cannot replace the glasses with the mirror image. It does not repeat the object in space because the position of the lenses is different.

This is because the glasses with a dark lens are no longer symmetrical and therefore, their mirror image is not the exact duplicate.
Let’s also do this with a molecule of dichloromethane (DCM), which is superimposable with its mirror image, and bromochlorofluoroiodomethane – a molecule nonsuperimposable with its mirror image.
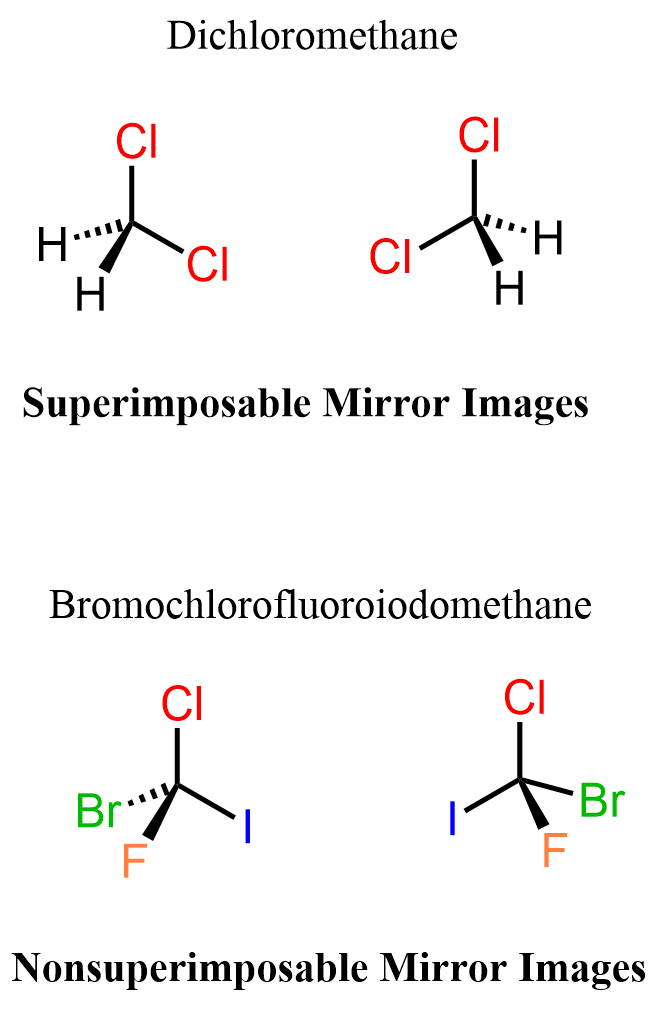
Let’s draw dichloromethane and its mirror image next to it.
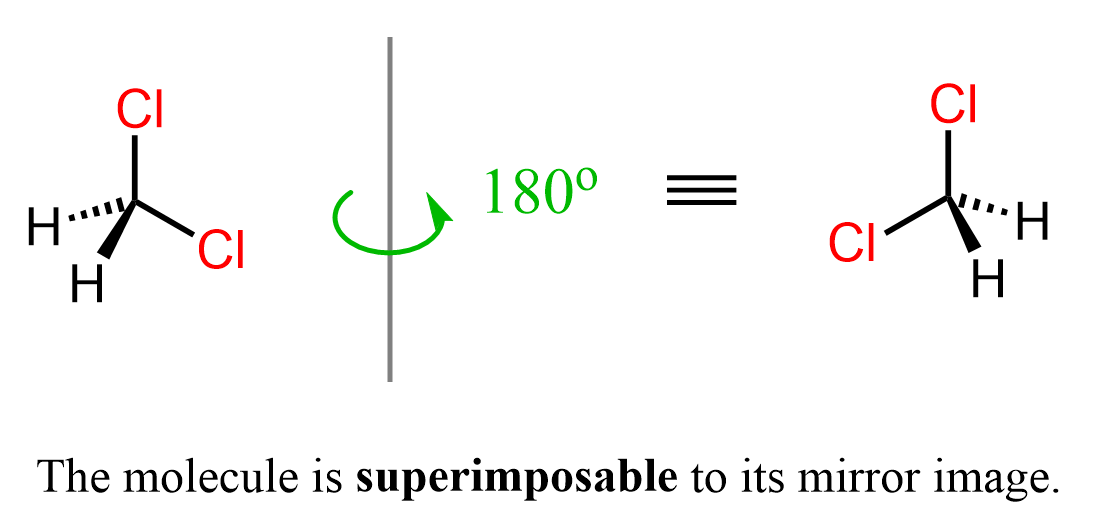
The representations are different, but they are superimposable because we can repeat, thus superimpose each of them by moving around the other one:
Now, if we replace one of the Cl, and both hydrogen atoms with bromine, iodine, and fluorine, a new molecule is obtained which is not superimposable with its mirror image.
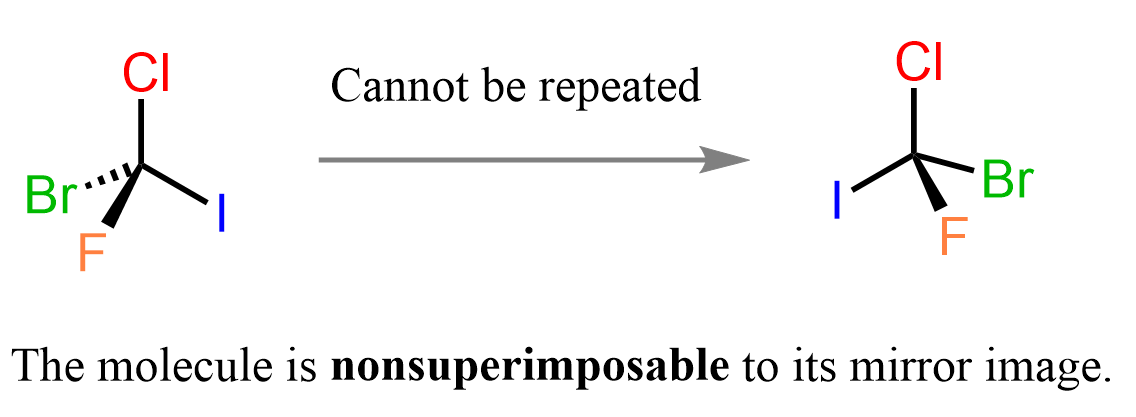
If you feel like this is some kind of trick and the objects are the same, try building the two molecules using a model, and you will see that you won’t be able to repeat identical representations – they are not superimposable.
Enantiomers and Nonsuperimposable Mirror Images
In chemistry, we classify molecules that are nonsuperimposable mirror images as enantiomers. Enantiomers are stereoisomers, which, remember, are isomers with the same connectivity but different spatial arrangement of atoms. For example, each pair of molecules represents stereoisomers because the connectivity of the atoms is the same, but some atoms have different spatial arrangements – they point in different directions relative to each other.

We cannot change the wedge and sash orientation of the atoms simply by moving them around like we did earlier with superimposable mirror images.
Notice that what is common in all these molecules is that they have at least one carbon atom that is connected to four other atoms. These are called chirality centers and it is the presence of four different atoms around the carbon that makes it unsymmetrical and thus nonsuperimposable to the mirror image of the molecule.
For example, if we did not replace one of the chlorines in dichloromethane with another atom, we’d have a carbon atom with only three different atoms, and the molecule would still be superimposable to its mirror image.
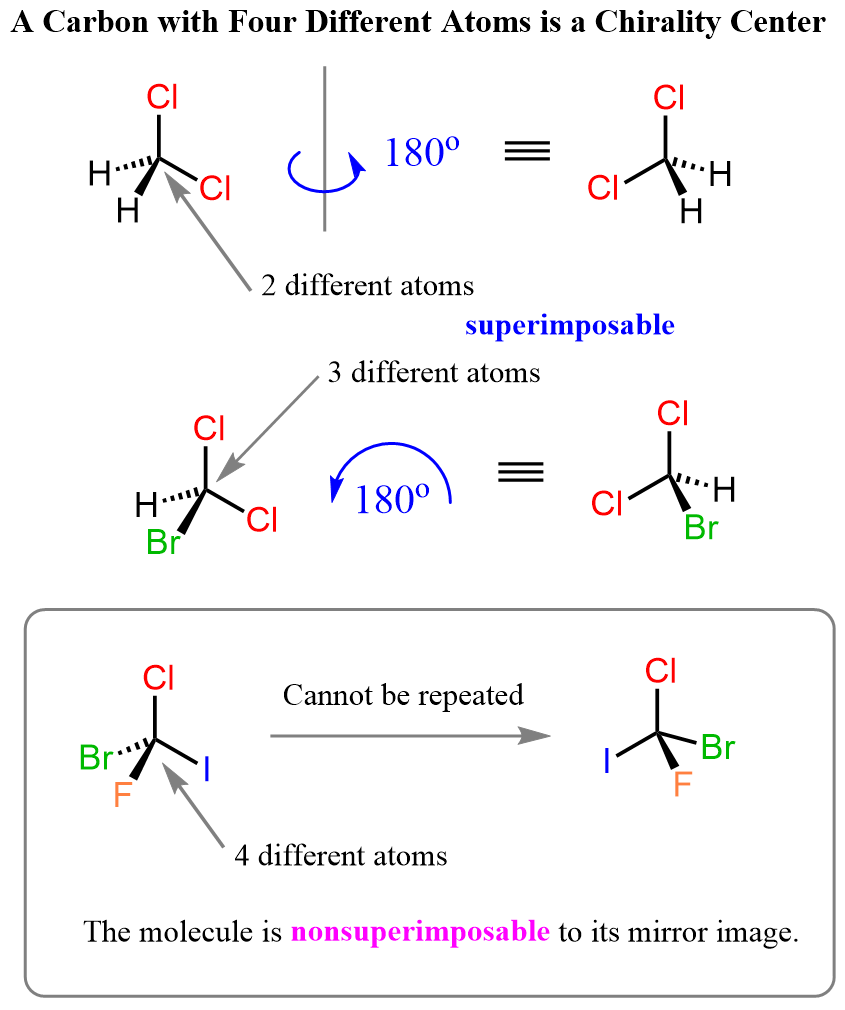
So, unless there is a carbon with four different groups connected to it, the mirror image of the molecule is superimposable to it. In other words, the molecule is symmetrical and its mirror image(s) represent the same species. On the contrary, a molecule is chiral when its mirror image is not superimposable with it, and in this case, the mirror images are enantiomers. Chiral molecules are not symmetrical (they are asymmetrical), and this is most often due to the presence of at least one carbon atom that bears four different groups.
Below are some examples of enantiomers, and you can check the next article for a more detailed discussion on those, as well as diving deeper into the types of isomers, determining their relationship, etc.
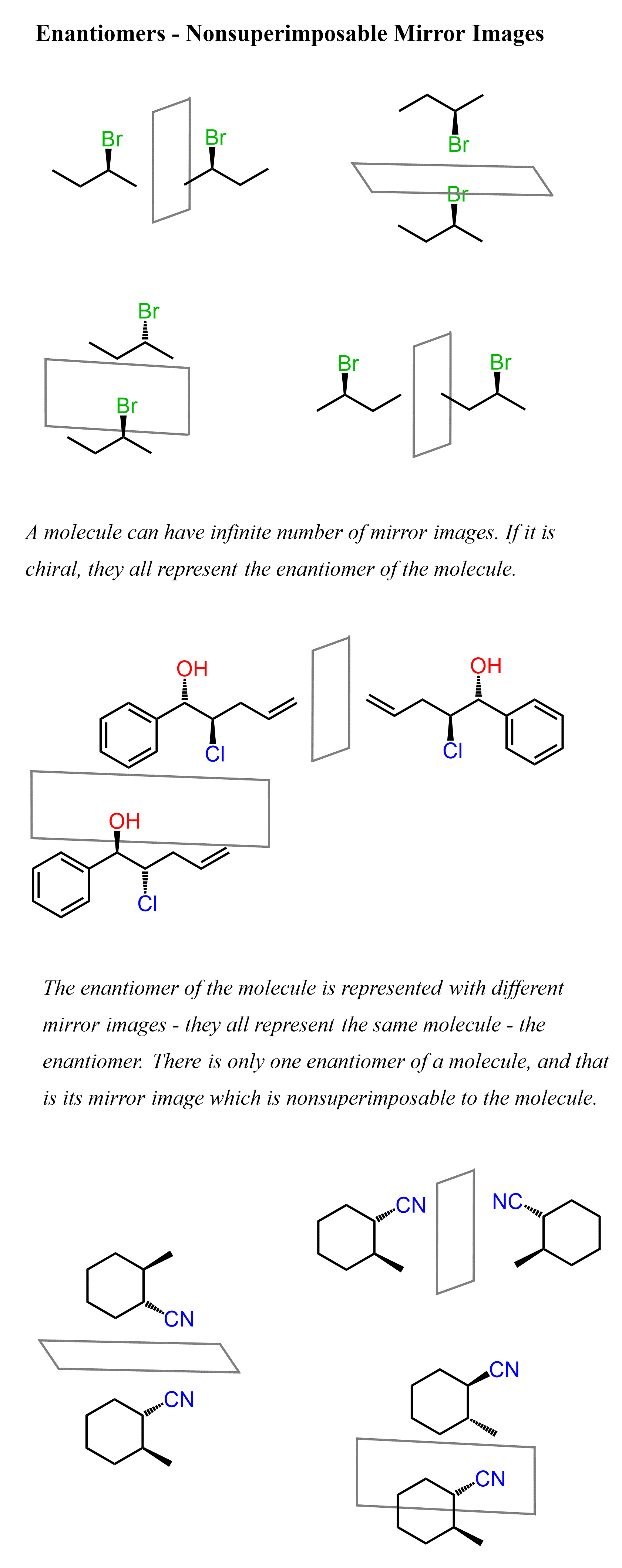
For each molecule, we have put a few mirrors to emphasize the fact that a molecule can have an infinite number of mirror images, but they all represent the same entity: if it is chiral, then the mirror image is nonsuperimposable to the molecule. Any chiral molecule has only one enantiomer, and it does not matter where and how many mirrors we place – they all represent the enantiomer of the molecule. Once again, a nonchiral molecule (also known as an achiral molecule) is superimposable to its mirror images.
You do not need to visualize a mirror next to the molecule and draw the reflection to see its enantiomer. In general, the easiest way to draw the enantiomer of a given molecule is to simply redraw the compound, replacing all dashes with wedges and all wedges with dashes.

However, attention! – You should not do both: you should not draw the mirror reflection and change the wedges and dashes together with it, since you will end up having the same molecule:

You should either keep the molecule as it is and change every wedge to a dash or you can place a mirror image anywhere (on the side, on the top, below, in front, or behind) next to the molecule and draw its reflection.
Check the article “Chirality and Enantiomers” for more details and examples on drawing the enantiomer of the molecule.
Enantiomers are not the only types of stereoisomers, as there are also diastereomers, but for now, make sure you have a good understanding of what nunsuperimposable mirror images are, so we can continue with chirality, enantiomers, and diastereomers in the upcoming posts.
Check Also
- How to Determine the R and S Configuration
- The R and S Configuration Practice Problems
- Chirality and Enantiomers
- Diastereomers-Introduction and Practice Problems
- Enantiomers vs Diastereomers
- Cis and Trans Stereoisomerism in Alkenes
- E and Z Alkene Configuration with Practice Problems
- Enantiomers vs Diastereomers
- Enantiomers, Diastereomers, the Same or Constitutional Isomers with Practice Problems
- Configurational Isomers
- Optical Activity
- Specific Rotation
- Racemic Mixtures
- Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
-
Converting Bond-Line, Newman Projection, and Fischer Projections
- Resolution of Enantiomers: Separate Enantiomers by Converting to Diastereomers
- Stereochemistry Practice Problems
- Stereochemistry Practice Quiz

