Dipole-dipole, London dispersion (also known as Van der Waals) interactions, hydrogen bonding, and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds. All of them are electrostatic interactions, meaning that they all occur as a result of the attraction between opposite charges, and which of these forces is present or predominates in a given compound depends on its functional groups.
Ionic Compounds
Back from general chemistry, we know from the structure of salts that oppositely charged particles tend to show very strong electrostatic interactions, and these are the ionic compounds. For example, sodium chloride (NaCl) is one of the first ionic compounds we talk about in general chemistry. Among other interesting features and applications, we learn that it has a melting point of 801 °C, which is extremely higher than the typical range for organic (covalent) compounds.
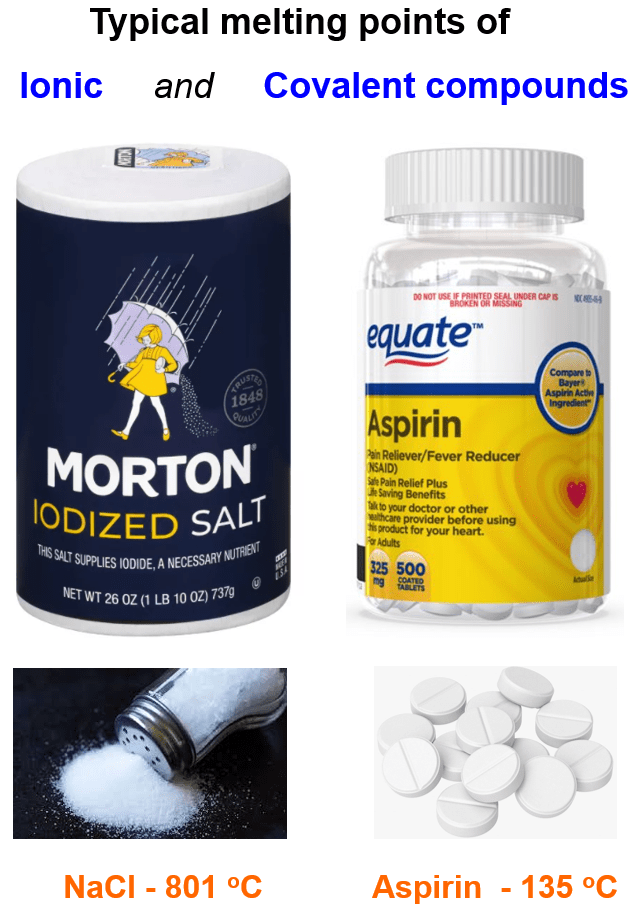
What is important here is that we are not talking about the interaction of one Na+ and Cl+ ion together. Unlike covalent bonding, ionic bonds are not directional and specific between given atoms, but rather they form a lattice as an interaction among the entire cluster of ions:

In other words, there is not really such a thing as one bond between two ions/atoms like in covalent compounds, and that’s why they need a lot of thermal energy to break these interactions and therefore, have high melting points.
We will discuss the relationship between intermolecular forces and physical properties later, but for now, keep in mind that the stronger these interactions, the higher the melting and boiling points are.
Now, most organic compounds are not ionic, and therefore, we will focus more on the intermolecular interactions between the covalent compounds.
Dipole-Dipole Interactions
To understand the nature of dipole-dipole interactions, remember that when two atoms with different electronegativities are connected, we have a polar covalent bond, and the shared electron pair of the covalent bond is not in the middle of the two atoms. There is a higher density towards the more electronegative element and as a result, both elements have partial charges. Mainly, the more electronegative atom bears a partial negative, and the other atom has a partial positive charge, which are indicated by the delta plus (δ+) and delta minus (δ-) symbols:

The direction and magnitude of polarity in a bond are given by the dipole moment, which is indicated by an arrow. The arrow starts from the less electronegative element and points toward the more electronegative element, as shown above.
How do I know if it is Ionic or Polar Covalent?
Polar covalent bonds and dipole moment originate when there is a significant difference in the electronegativities of the two bonded atoms. Now, the ionic bonding occurs when the difference in electronegativities is very large and one of the atoms completely takes the bonding electrons.
Remember the trends of electronegativity; it increases across a row of the periodic table (left to right), and goes up a column of the periodic table. Here is a table with the electronegativity values of the most commonly encountered elements in organic chemistry:

So, you may wonder what the “large” and “little” differences are, and how large the electronegativity difference should actually be to consider a bond polar or ionic. This often causes confusion; however, it is accepted that a bond is polar when the electronegativity difference between two atoms is ≥ 0.5 units. Anything with less difference is considered a nonpolar bond.
For example, alkyl halides are the compounds you will see a lot in organic chemistry, particularly in substitution and elimination reactions. Now, while the C-F will by all means be considered a polar bond (1.5-unit difference), the C-I bond does not fit in this definition (0.2-unit difference). However, the C-I bond is very reactive, and we put partial charges on the bonded atoms. This is because of polarizability, which is the characteristic of how the electron cloud around an atom responds to changes in the electronic environment.
We will discuss this later, but as far as the carbon-halogen bonds and dipole moments, keep in mind that these are all considered polar covalent bonds. The C-C and C-H bonds are nonpolar and the least reactive in traditional ionic reactions.
You may also wonder why we care about the dipole moment and what its use is in organic chemistry. There are two main aspects here: the physical properties (mainly melting and boiling points) and the reactivity of organic molecules having a dipole moment. While the physical properties can be mostly covered in one article, the substitution and elimination reactions are entire chapters on their own.
· Nucleophilic Substitution Reactions
· Elimination Reactions
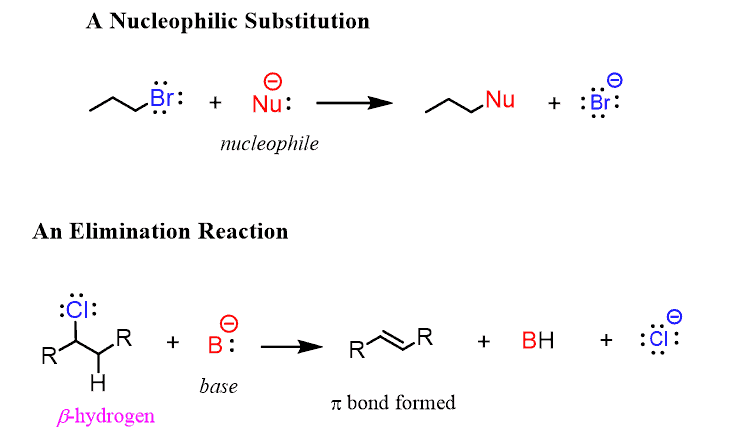
A good starting point for these is learning about the alkyl halides here.
And one more thing about the dipole moment and polarity. Just because there are dipole moments in the molecule, it does not necessarily mean it is a polar molecule. To determine the molecular polarity, we also need to consider the geometry of the molecule. This brings up another topic on its ow,n and therefore, a separate article is dedicated to it:

Electrostatic Maps
The electron density of a polar covalent bond can also be shown with electrostatic maps. These are simply color-coded clouds where the blue usually corresponds to the most electron-deficient region, and the red to the most electron-rich region.
For example, in fluoromethane, shown above, the fluorine atom has the highest electron density, and the carbon atom has less, so this is how the electrostatic map would look:

As expected, the electron-rich F atom is in the red region since, being more electronegative, it pulls the electron density and thus makes the carbon atom electron-deficient.
London or Van Der Waals Forces
We know that nitrogen is a gas, and in fact, it makes up about 79% of the atmospheric gases. However, at temperatures below -196 oC (–320°F), it can be kept as a liquid in sealed containers or Dewar flasks. Although if not trained, it is very dangerous to work with, you may find a lot of videos of  people doing experiments with liquid nitrogen. Things like putting an orange, leaves, or a Coke to freeze before crashing it are a few to mention.
people doing experiments with liquid nitrogen. Things like putting an orange, leaves, or a Coke to freeze before crashing it are a few to mention.
Now, in terms of chemistry, what we understand from the fact that nitrogen can be turned into a liquid is that there must be some type of intermolecular interactions that hold the molecules together. However, nitrogen is an absolutely nonpolar molecule since it consists of two identical atoms. So, what interaction are we talking about? We know that nonpolar compounds lack dipole-dipole interaction; there is no hydrogen, so it cannot be hydrogen bonding either.
Albright then, as the title said, these are the Van Der Waals forces, also known as London dispersion forces.
These are the weakest intramolecular interactions and occur as an electrostatic interaction of temporary dipole moments formed in the molecule right at the time when they get close enough. What happens during this instant is that the electron density of the molecule is distorted because of the attracting and repelling forces between the nuclei and electrons of the two molecules.

These interactions create a tiny instantaneous dipole moment as a result of the charge imbalance. The temporary opposite charges in different molecules align, then affecting the physical properties. The London dispersion forces are characteristic of small molecules only. They apply equally to larger organic and inorganic compounds:

We will discuss the effect and magnitude of London forces on the physical properties of organic alkanes in the next article.
Hydrogen Bonding
Hydrogen bonding is another type of intermolecular electrostatic interaction that occurs between a hydrogen atom bonded to an electronegative atom, such as O, N, or F, is attracted to a lone pair of electrons on an atom in another molecule. To emphasize this one more time; hydrogen bonding is not a covalent bond within the molecule, but it is a specific type of dipole-dipole interaction. The basis of it is the attractive forces between the partial positive charge of the hydrogen and an electron pair of the other molecule. Hydrogen bonds are shown with dotted lines connecting a lone pair with the hydrogen.
Let’s, as an example, compare the covalent bond between the oxygen and hydrogen in the same molecule of ethanol vs the hydrogen bonding of the hydrogen with the oxygen atom in another molecule of ethanol:

What happens is that the hydrogen atom connected to the more electronegative oxygen atom bears a partial positive charge (δ+) because of the oxygen pulling the electron density through induction. This δ+ then interacts with a lone pair of the more electronegative oxygen atom of another molecule of ethanol.
Hydrogen bonding is very common in nature, starting from water and going to complex biological systems like DNA and proteins. It is the hydrogen bonding that is responsible for the complementary nature of DNA nucleobases and thus, the principle of genetic coding.

As expected, hydrogen bonding affects the physical properties, and we will discuss this by looking at alcohols, ethers, and different amines in the next post.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- Bonding Patterns in Organic Chemistry
- How to Determine the Number of Lone Pairs
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz

Very useful material, thank you
You guys are so expert and talented to provide the best intellectual useful materials on the scope of chemistry studies love you guys much appreciation to your organisation ,bird , management and coordinator
Thanks, Johnson, for such encouraging words!