In this post, we will talk about the melting and boiling points of organic compounds and their correlation with intermolecular forces such as dipole-dipole, London dispersion (also known as Van der Waals) interactions, and hydrogen bonding. We discussed these infractions in the previous post, so today, the focus will be more from the perspective of physical properties.
Let’s put the relative strength of intermolecular interactions right before we get started. In general, for compounds of approximately the same molecular mass, you can follow this trend for the strength of intermolecular interactions:

We will discuss the strength and effect of each interaction typical of covalent compounds below. The ionic bonding is the strongest intermolecular interaction characteristic of inorganic compounds, which, as a result, have very high melting points. These, however, are not so relevant in organic chemistry, and therefore, we won’t focus on them as much in this article.
Boiling Point and Dipole-Dipole Interactions
We mentioned in the previous post that stronger intermolecular interactions increase the boiling and melting points, but how exactly they affect the physical properties might be your next question.
The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals the atmospheric pressure. In other words, we can say it is the temperature at which there is so much of the compound evaporated that it creates a pressure equal to the external pressure. In a simpler perspective, let’s say that it is the temperature when the liquid turns into a gas, even though this process can occur at a large scale of temperature and pressure combination.
Now, to turn into the gas phase, the molecules should overcome the intermolecular interactions and escape the liquid surface, and the stronger these interactions, the harder it is for the molecules to overcome them.

Let’s first discuss the effect of dipole-dipole interactions on the boiling point of organic covalent compounds. Dipole-dipole interactions are not so strong (weaker than ionic and covalent bonding).
However, in reality, we never deal with two or three molecules, but rather we work in the mole scale. And this means, despite being weak, dipole-dipole interactions contribute significantly to the physical properties of compounds when thousands or millions of molecules chain together through this electrostatic interaction. Let’s show this with an example of a common organic solvent, acetone:
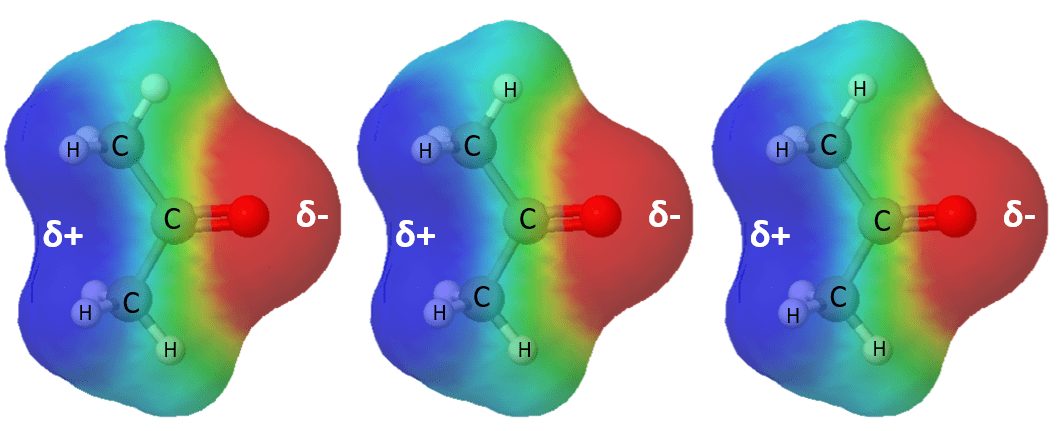
And now let’s compare the boiling points of Isobutylene and acetone. They have a similar structure and only differ in that one of the carbon atoms is replaced by an oxygen. This difference, however, introduces a polar covalent bond and therefore, dipole-dipole interactions, which elevate the boiling and melting point of the compound:

Isobutylene, on the other hand, is a nonpolar molecule lacking dipole-dipole interactions since it only consists of C-C and C-H bonds.
Boiling Point and Hydrogen Bonding
You can check the previous post for more details about the hydrogen bonding. In short, it is another type of intermolecular electrostatic interaction that occurs between a hydrogen atom bonded to an electronegative atom, such as O, N, or F, that is attracted to a lone pair of electrons on an atom in another molecule.

As expected, hydrogen bonding affects the physical properties, which we can see, for example, by comparing the boiling point of ethanol shown above and dimethyl ether. These two are constitutional isomers, meaning they have the same chemical formula and therefore, molecular mass.
However, despite having the same molecular mass, these compounds have completely different physical and chemical properties:

The reason for this difference is the lack of hydrogen bonding in the ether since it lacks hydrogen atom(s) connected to an electronegative atom.
Similar behavior can be seen when comparing the boiling points of three isomeric amines: Trimethylamine, Ethylmethylamine, and Propylamine. These are examples of tertiary, secondary, and primary amine,s which are defined based on the number of alkyl groups connected to the nitrogen.

Notice that the boiling point increases as we go from the tertiary to the primary amine as a result of increasing the number of hydrogen bonds per nitrogen. Propylamine has two hydrogens connected to the nitrogen, and each can make a hydrogen bond with a neighboring nitrogen atom from another molecule. This makes a stronger intermolecular interaction and therefore, more energy is needed to break it pushes the molecules to the gas phase. The tertiary amine, on the other hand, has no hydrogens and boils at a lot lower temperature.
London or Van Der Waals forces
Another factor that influences the boiling point is the surface of the molecule. The larger this surface, the stronger the intermolecular interactions, and thus, the higher the boiling point. This can be seen by comparing the boiling points of pentane, 2-methylbutane, and 2,2-dimethylpropane:

Again, all three are constitutional isomers and have identical molecular weights. In addition, they also lack dipole-dipole interactions since there is no polar covalent bond present.
However, the boiling point decreases quite significantly as we move towards the more branched isomers. And this is a demonstration of a direct relationship between the surface area and the boiling point. Pentane is unbranched and provides a large surface for intermolecular interactions. The 2-methylbutane has one substituent, so it is a little more branched than pentane. This reduces the surface for intermolecular interactions and lowers the boiling point by about 8 oC. The highly branched 2,2-dimethylpropane, on the other hand, lacks this surface interaction and has the lowest boiling point.
The analog of this can be the regular stacking of regular vs crumpled paper sheets.

To summarize, branching tends to decrease the boiling point of alkanes with the same molecular weight.
All these examples demonstrate the importance of the molecular surface in intermolecular interactions, which directly affect the boiling point of a compound. However, you might have noticed one important thing missing here.
What interaction are we talking about?
We just said that the molecules are nonpolar and therefore lack dipole-dipole interaction, so what type of interactions increase the boiling point with a larger surface?
These are the Van Der Waals forces, also known as London dispersion forces, which are the weakest intramolecular interactions and occur as an electrostatic interaction of temporary dipole moments formed in the molecule right at the time when they get in a close enough distance:

You can read more about Van Der Waals forces in this article.
Melting Points
Just like we simplified the boiling point to explain the effect of intermolecular interactions on it, let’s formulate that the melting point of a compound is the temperature at which it is converted from the solid to the liquid phase.
In general, the melting point of compounds with similar molecular weight increases with stronger intermolecular interactions.
For example, pentane has a very low melting point compared to butanal since it only relies on London dispersion forces, while butanal contains a polar C=O bond and therefore exhibits dipole-dipole interactions:

Potassium tert-butoxide, being an ionic compound, has the highest melting point. 1-butanol is the second because of the OH group and thus, hydrogen bonding. Remember, the order of increasing intramolecular interactions in covalent compounds:
Symmetry and Melting Point
Aside from the intermolecular interactions, however, the melting point also depends on how the molecules are packed or arranged in the solid form. The more symmetrical they are, the better they pack and form a perfect crystal lattice, which results in a higher melting point. So, as the molecules fit tightly, more energy is required to break the lattice and melt them apart.
Let’s go back to the examples we discussed for the boiling point. Remember, the boiling point increases with less branching because of the increased surface area. So, pentane had a higher point than 2,2-dimethylpropane; 36.1 oC vs 9.5 oC.
Interestingly, the pattern is not observed for the melting points. 2,2-dimethylpropane has a higher melting point since it is more symmetrical than pentane, and when in the solid phase (before melting) its molecules are better packed.

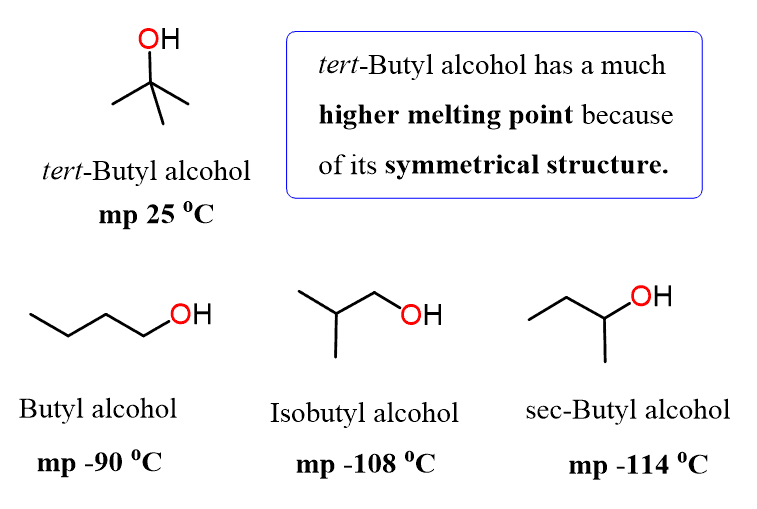
We can also see the effect of symmetry by comparing the melting point temperatures of the butanol isomers. All of these are constitutional isomers capable of hydrogen bonding. However, compared to the other isomeric alcohols, the tert-Butyl alcohol has a much higher melting point because of its symmetrical structure and therefore, compact packing in the solid phase:
And another great example to illustrate the importance of packing and molecular symmetry is the comparison of the melting and boiling points of cis– and trans alkenes. In general, trans alkenes have a higher melting point.
For example, despite being nonpolar, the trans isomer of 1,2-dichloroethane has a higher melting point (−50 oC) than the cis isomer (−80 oC) because of higher symmetry, which allows for compact packing in the solid phase.
In contrast, the cis isomer is a polar molecule with a higher boiling point (60 oC vs 48 oC ) because of the net molecular dipole moment and intermolecular dipole-dipole interactions.

Summarizing it, remember that given the same functional groups, the boiling and melting points would naturally be expected to increase with the molecular mass (size) of the molecule. Also, stronger intermolecular interactions presume higher boiling and melting points. However, for the melting point, you also need to consider the factor of symmetry. More symmetry means tighter packing in the solid phases and therefore, a higher melting point.
In the next article, we will also discuss the effect of intermolecular interactions on the solubility of organic compounds.
Check Also
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- Bonding Patterns in Organic Chemistry
- How to Determine the Number of Lone Pairs
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz



It’s really helpful
Good to hear that!
Thank you ,This is helpful to studies .
Thanks, Manuthi.
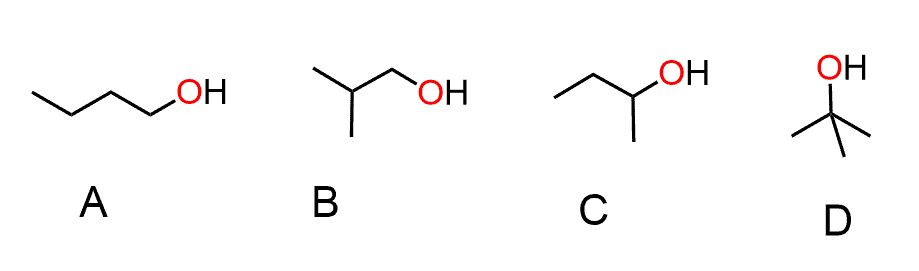
Hi. May I ask is the boiling point of sec-butyl higher or lower than the isobutyl and why? Thank you
Hi there,
If it’s about the respective alcohols, you can do a quick Google search. To explain the differences in their melting points, refer to the trends mentioned in this article.