Question: Why do we use curved arrows?
Answer: We use them to keep track of electrons.
Curved arrows are crucial in organic chemistry, and using them correctly is essential for mastering the subject. In fact, it is like the operating system of organic chemistry, so the sooner you master the principle behind it, the easier it will be for you to understand many concepts in organic chemistry.
Let’s start!
Every curved arrow has a head and a tail for showing the flow of electrons from a high electron density to a low electron density center. The arrow must start from the middle of a lone pair or a covalent bond.

For example:

In this reaction, the electrons move from the Cl to the carbon, and as a result, a new bond is formed.
In the next example, the curved arrow shows the movement of the electron pair shared between the carbon and Br (that is, from the C-Br bond) to the Br:

Therefore, this represents the breaking of the σ bond.
If we started the arrow from a π bond, then that would indicate breakage of the π bond. For example:

The key observation here is that curved arrows show the flow of electrons. And that is the first and most important thing you need to remember about curved arrows:
Curved arrows show the movement of electrons. Therefore, any curved arrow mechanism starts from a lone pair of electrons or a covalent bond.
Do not start them from a positive charge or a plain atom with no lone pairs:

Starting from a negative charge is also acceptable.

Curved arrows in resonance structures
There are two main areas where curved arrows are used. The first one is their use in resonance structures, and the second is their use in demonstrating the mechanisms of organic reactions.
The big difference between these two is that in resonance structures, the connectivity of atoms stays the same. This means that resonance structures represent the same entity, only with a different electron distribution.

In fact, even the electrons do not move in resonance structures, and we are simply showing them as such to keep track and explain certain properties and reactivity of compounds.
The main implication of the fact that resonance structures represent the same molecule/ion is that you cannot break any σ bonds, as this would change the connectivity of atoms, hence different molecules would form. So, when initially we said that curved arrows must start either from a lone pair of electrons or a covalent bond, this statement is narrowed down for resonance structures: Curved arrows in resonance structures must start either from a lone pair or π bonds.

In general, the following two rules must be followed when drawing resonance structures:
1) Do not exceed the octet on 2nd-row elements.
2) Do not break single bonds

There is a lot more about this in the following post, “Rule for Drawing Resonance Structures”, so feel free to read the material and then continue to the next part.
Curved arrows in organic reaction mechanisms
Curved arrows are a formal notation to help us understand the electron flow in organic reactions. This makes it easier to track the bonds forming and breaking during the reaction, as well as visualize and explain more advanced features, such as the region and stereochemistry of certain reactions.
The electrons always flow from a high electron density region to a low electron density region. The molecules with a high electron density are nucleophiles – i.e., love nucleus. A molecule with a low electron density is classified as an electrophile – i.e., loves electrons.
The reaction below illustrates nucleophilic substitution, one of the most important types of organic reactions that you will learn later in the semester:
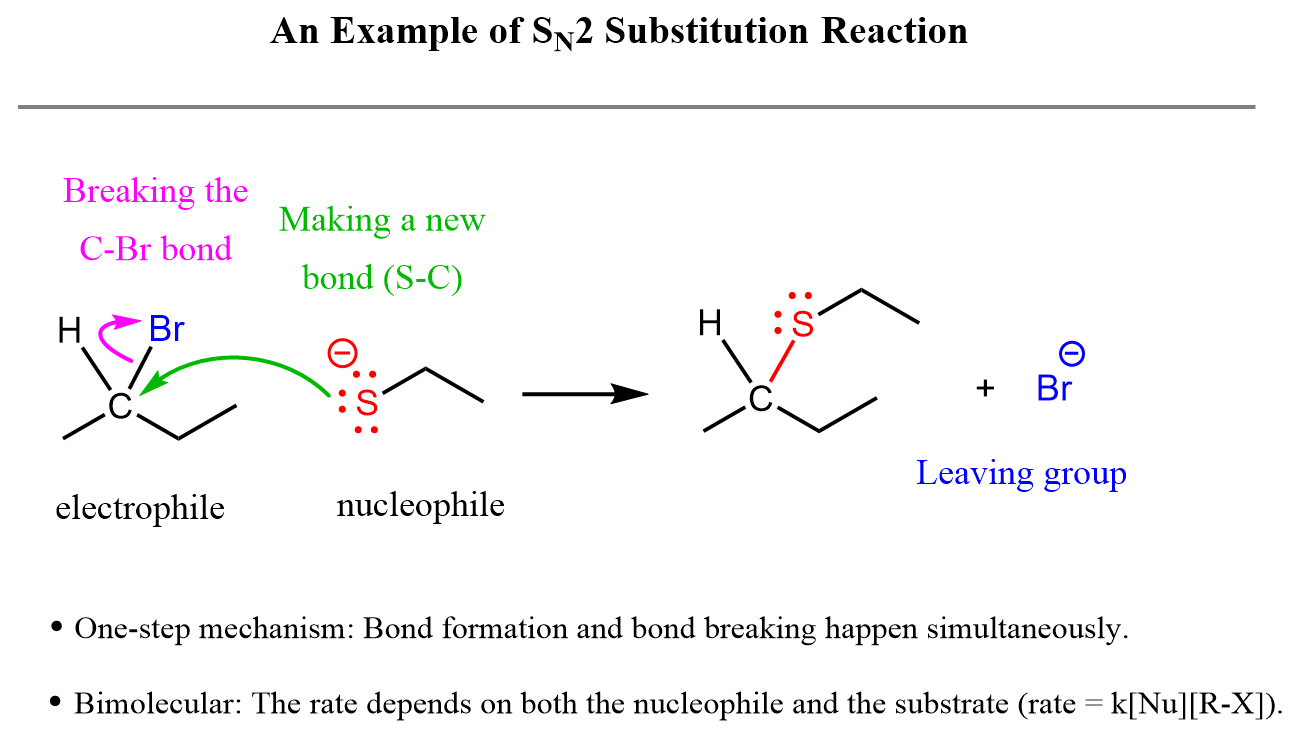
The arrow starting from the lone pair on sulfur and pointing to the carbon shows the formation of a new covalent bond between them. The second arrow, drawn from the middle of the C-Br bond, indicates that this bond is breaking and that both electrons are moving to the bromine atom.
Another important class of reactions that we can consider for learning the curved arrows is the acid-base reactions:
For example:

Here, the hydroxide ion is the base, and it attacks the proton connected to the carbon. So, this curved arrow shows a bond forming between the oxygen and the hydrogen. The second arrow indicates breaking the bond between the hydrogen and the carbon, as otherwise, the hydrogen would have left with two bonds, which is not possible.
Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.
Molecular Representations Quiz




Brilliant! Hugely helpful….thankyou!
Glad it was helpful, Jenna.
Hello just want to I think there is an error in practice question 2 part (a) because another alkyl group is added for resonating structure which makes the no of atoms more in second RS.
Thanks for letting me know, Josh – fixed.
There is an error in the last part:
Here, the hydroxide ion is the base and it attacks the proton connected to the nitrogen(should be “carbon”)
Fixed. Thank you!
Hi, just wanted to mention that there is another error in the same paragraph:
“The second arrow indicates breaking the bond between the hydrogen and the nitrogen”
where nitrogen is mentioned instead of carbon!
Oh man, that paragraph… Thank you – fixed!