What are Resonance Structures
When switching from general to organic chemistry, showing molecules as structures rather than simple formulas becomes one of the first things and priorities you need to learn. Lewis structures are essential for this as they show all the bonds and electrons in the molecule.

For some molecules, it is possible to have more than one Lewis structure accurately presenting the bonds and electron features in general. For example, acetone can be represented with two Lewis structures since the connectivity of atoms stays the same and only the electron distribution is changed.

As long as we keep the atoms connected the way they are, we are free to change the electron distribution in the molecule and show it in more than one form.
These two structures are called resonance structures or resonance forms of the same compound.
The charges are called formal charges, and you can read about them here.
Resonance structures are separated by a double-headed arrow. Do not use two arrows, as they are used for equilibrium reactions.

Notice again that only the arrangement of electrons is different in resonance structures – atoms have the same connectivity. And that is the definition of identical compounds – they must have the same connectivity of atoms.
Curved arrows in Resonance structures
Let me mention this before we start: you are going to see and use curved arrows every day you deal with organic chemistry, so you need to like learn them the sooner the better.
Every curved arrow has a head and a tail for showing the flow of electrons from a high electron density to a low electron density center.

The arrow shows the direction of electron flow:

Pay attention that the tail starts from the middle of a lone pair or a bond, and the head stops on a specific atom or the middle of a bond:
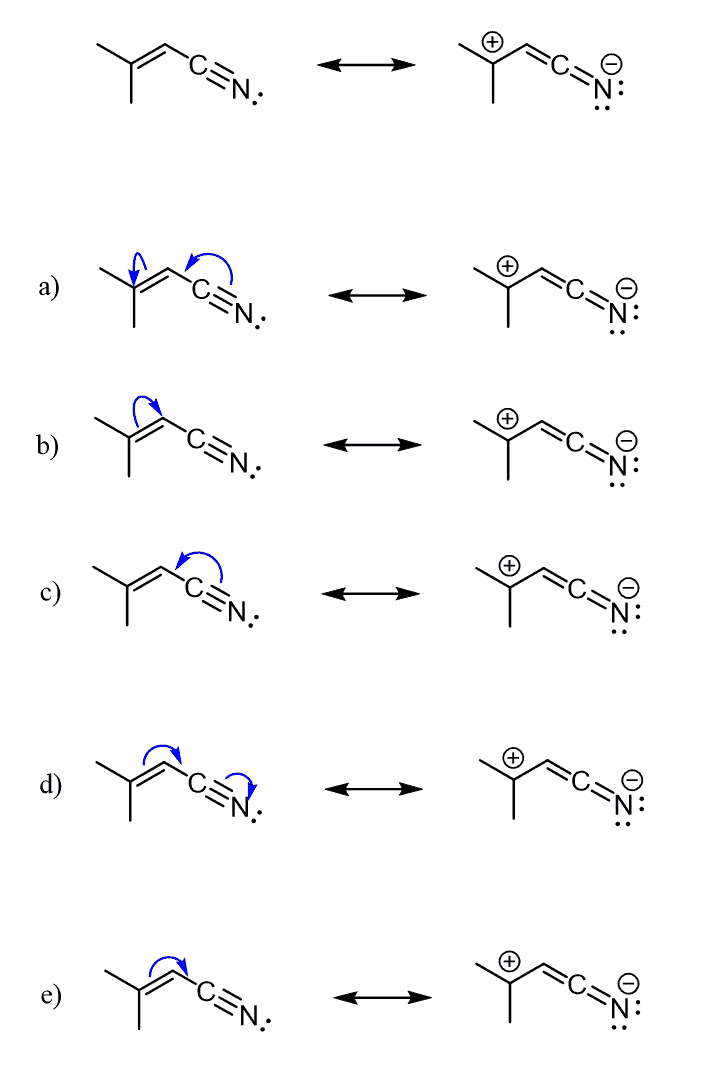
Here is the first and most important thing you need to remember about curved arrows. Regardless of whether the arrow starts from a lone pair or a π bond, it indicates a pair of electrons since the bond is also a pair of electrons.
Therefore, remember – Curved arrows show the movement of electrons.

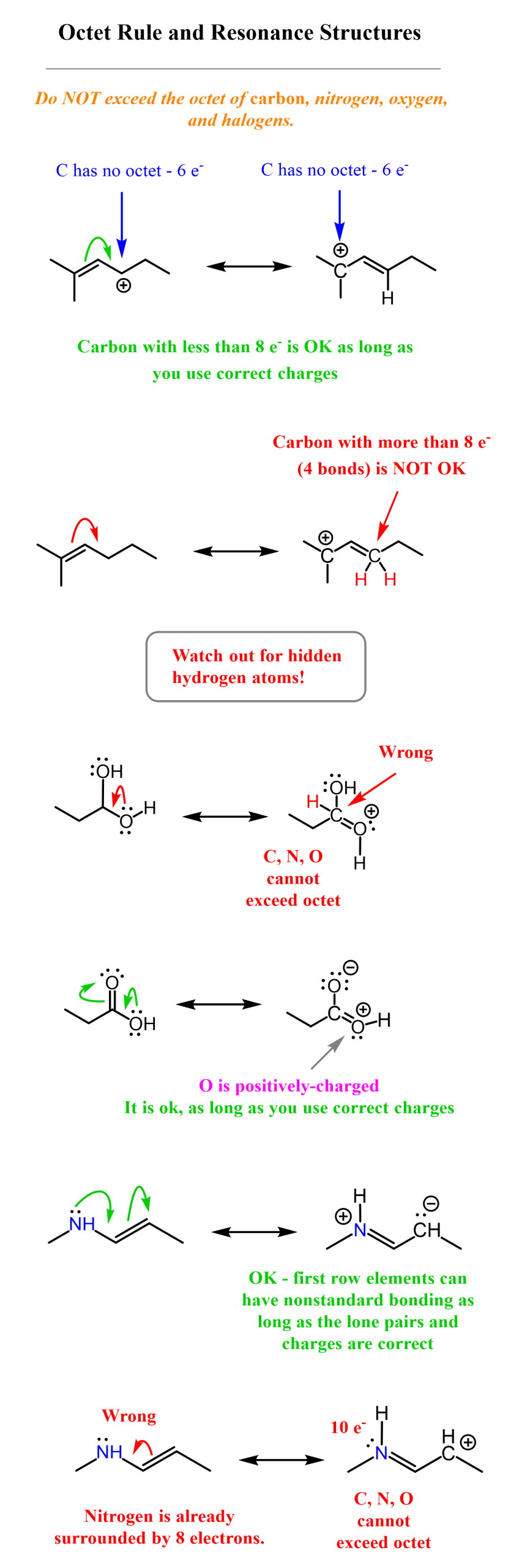
Do not start curved arrows from a positive charge or a plain atom with no lone pairs.

Starting from a negative charge is also acceptable (check with your instructor to be sure).
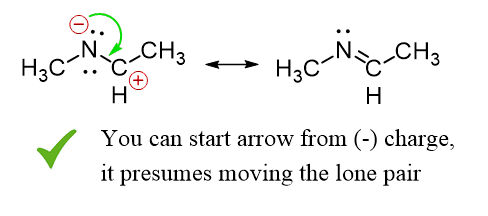
Resonance hybrid and movement of electrons
I know that I have just told you that curved arrows show the movement of electrons, but I also need to tell you something that goes against this.
Even though we use curved arrows and move the electrons around in resonance structures, you need to know that the electrons do not actually move in the sense of jumping from one atom to another, as we show them in resonance structures.

In reality, the electrons are spread among the atoms (the electrons are delocalized), and none of the resonance forms is the correct representation of the molecule.
The accurate representation of the molecule is given by the resonance hybrid.

Having the resonance forms in brackets is to indicate that they represent one entity, which is the resonance hybrid where the charge (electrons) are spread over the two atoms.
You may wonder now – why do we then draw resonance structures and use curved arrows? And the answer to this is that some properties and reactions of molecules are better explained by the individual resonance structures, and we use them with curved arrows to keep track of electrons and explain these properties.
Think about a hybrid fruit nectarine. It is a mix of a peach and a plum, and to explain its color, texture, and taste, we refer to the individual fruits.

This, however, does not mean that the nectarine exists as a peach for some time and then turns into a plum. None of them is a correct representation of the nectarine, just like none of the resonance structures is the correct representation of the given molecule.
So, do the curved arrows show the movement of electrons in a resonance structure? The correct answer is no, in reality, they don’t, but on paper, yes, they do. Otherwise, they would have no meaning or purpose.
Rules for drawing resonance structures
Two must-follow rules when drawing resonance structures:
1) Do not exceed the octet on 2nd-row elements.
2) Do not break single bonds
Rule 1. The second-row elements (C, N, O, F) can only handle up to eight electrons because of their orbitals. And this means you should never place more than eight electrons on those, i.e., you must follow the octet rule.
They may have fewer than eight electrons, but never more. Read this post to refresh standard valences and formal charges in organic chemistry.

Rule 2: Do not break single bonds. The basis of this rule is that atoms must have the same placement in resonance structures otherwise, they are not resonance structures but rather different molecules.

You can only move electrons in writing resonance structures if it does not change the way the atoms are connected. So, one way of drawing a resonance structure above would be starting the arrow from the lone pair and then breaking the π bond:

One good pattern to remember is that resonance structures involve a π bond, one way or the other. It is either making a bond or breaking a bond or both.
Therefore, whenever asked to draw a resonance structure(s), look for a π bond. You can’t have resonance structures without having a π bond involved.

If there is no π bond, then it would have to be formed in the new resonance structure.
I have also written a separate post on the rules for drawing resonance structures, where we address them in more detail and point out some common mistakes made by students on exams. Take some time to check that out as well.
Resonance Stabilization
You have probably noticed that the formal charge appears on different atoms depending on the resonance structure:

Essentially, the more resonance structures the molecule has, the more atoms handle the formal charge(s), which stabilizes the molecule. These structures, however, are not equal in terms of their stability, thus in their contribution to the resonance hybrid. The one with no formal charges is generally the most stable, also known as the major resonance contributor.
Compare the ethoxide ion with the acetate ion. They both have a negative charge on oxygen, but the acetate ion is a lot more stable because the electrons (negative charge) are spread over/delocalized between the two oxygen atoms, which helps each other handle this charge.

This is resonance stabilization – a molecule with more than one resonance form is resonance stabilized, and the more resonance structures it has, the more stable it gets.
Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.
Molecular Representations Quiz