You have already learned that carbon likes to have four bonds as it fulfils the octet without the “need for thinking” about lone pairs or formal charges. So, that is the very beginning of bonding patterns in organic chemistry – never show a carbon with five bonds.
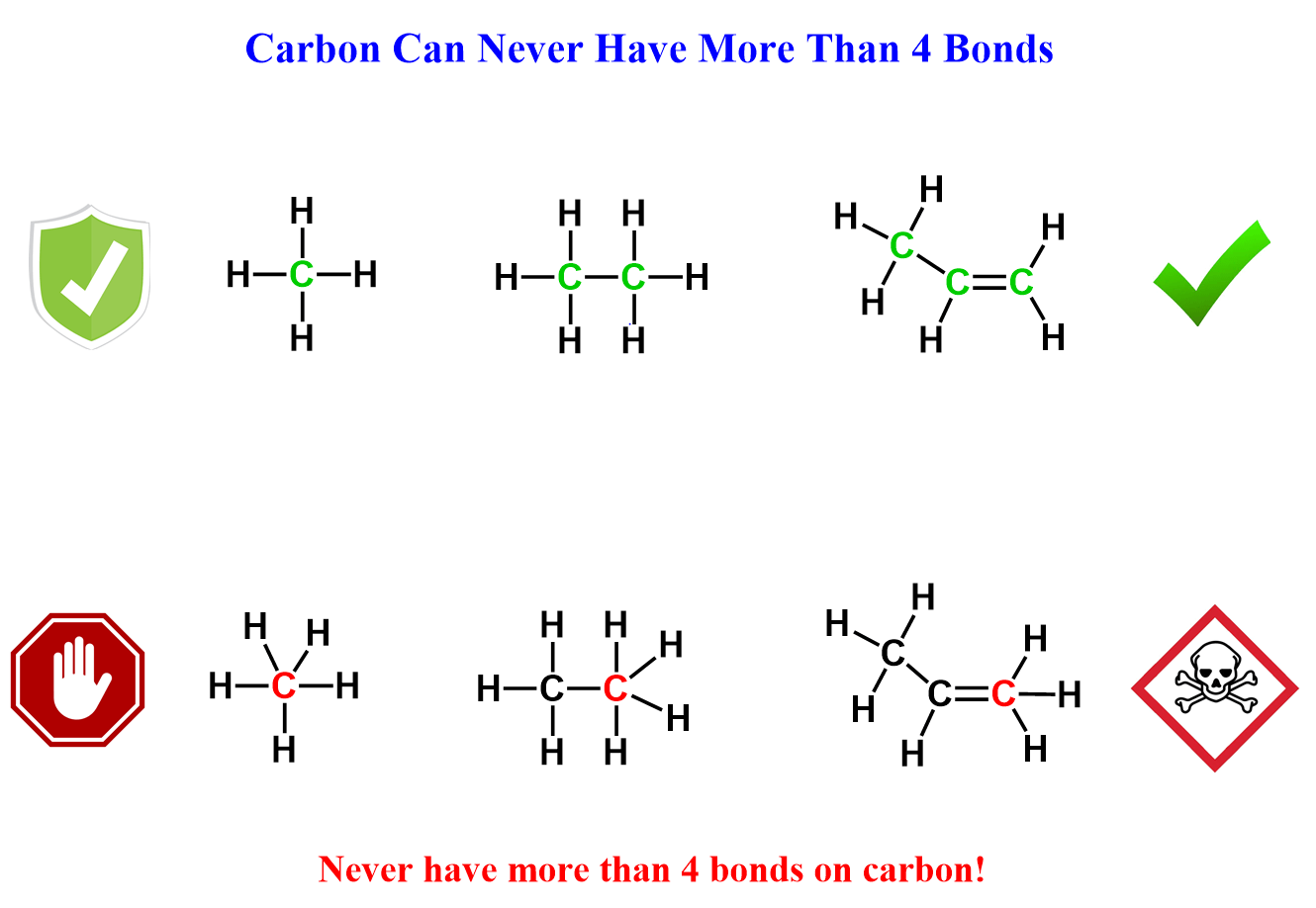
As much as it looks simple, there are times when you are going to make a mistake and show carbon atoms exceeding the octet because of hidden hydrogens in bond-line structures. However, this will take some time and practice, and you will be past the stage of counting all the bonds around every carbon, and drawing correct bonding patterns will be the routine habit in your organic chemistry class.
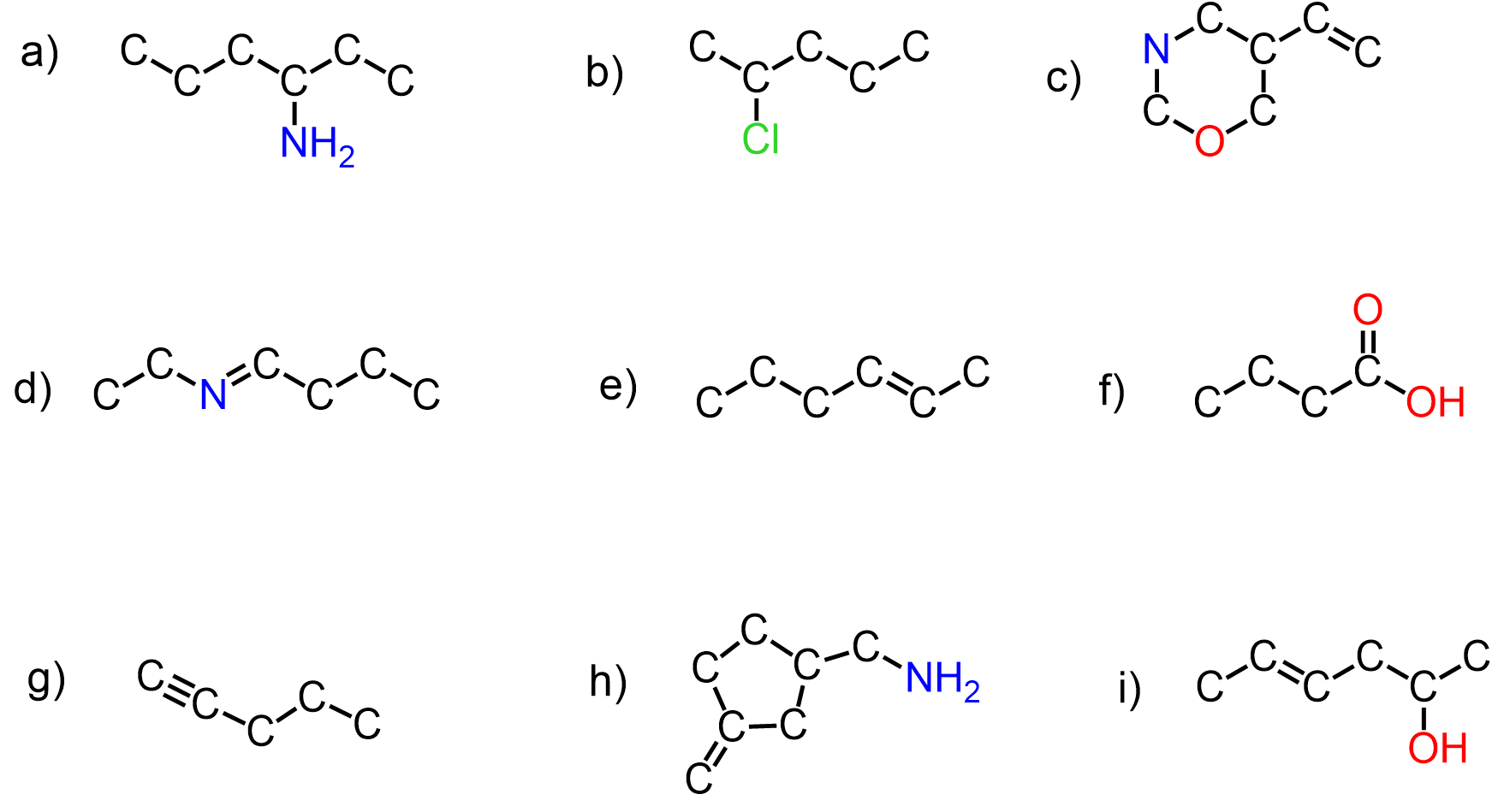
Aside from carbon, remember also the standard number of bonds for nitrogen, oxygen, and halogens, as these are the atoms most often seen in organic chemistry. In their neutral form, carbon, nitrogen, oxygen, and halogens have the following pattern of bonds and lone pairs in organic chemistry:
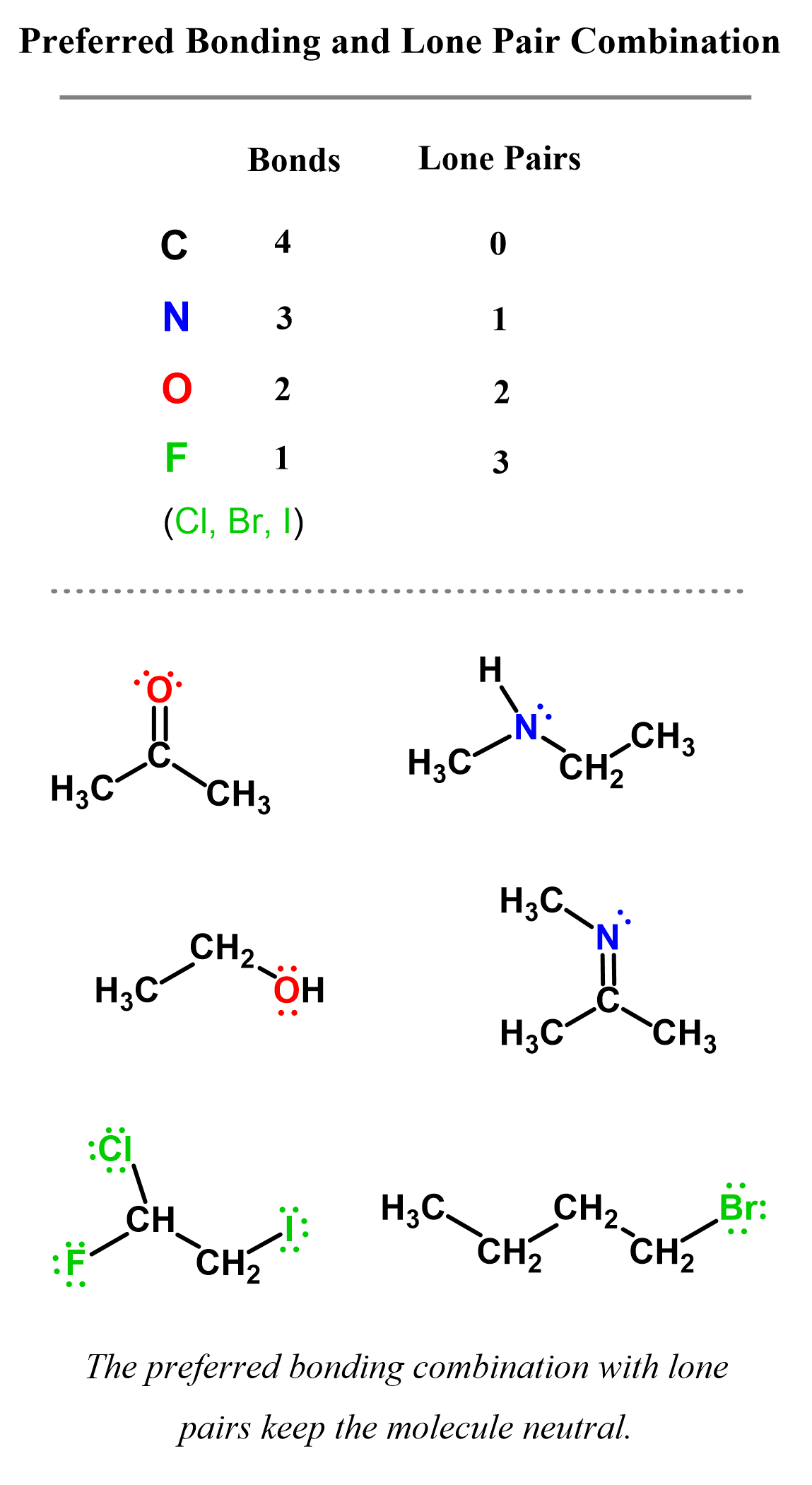
Let’s now see when these atoms are charged and what is the role of lone pairs is in the structure of those ionic species.
Formal Charges
The next factor is formal charges, which become relevant when the atom is off its standard bonding pattern. So, for carbon, that would be when it has three bonds. This can be three bonds with or without a lone pair.
In the first case, the carbon is negatively charged, and we have a carbanion, whereas a carbon with three bonds and no lone pairs is positively charged, thus the name carbocation:
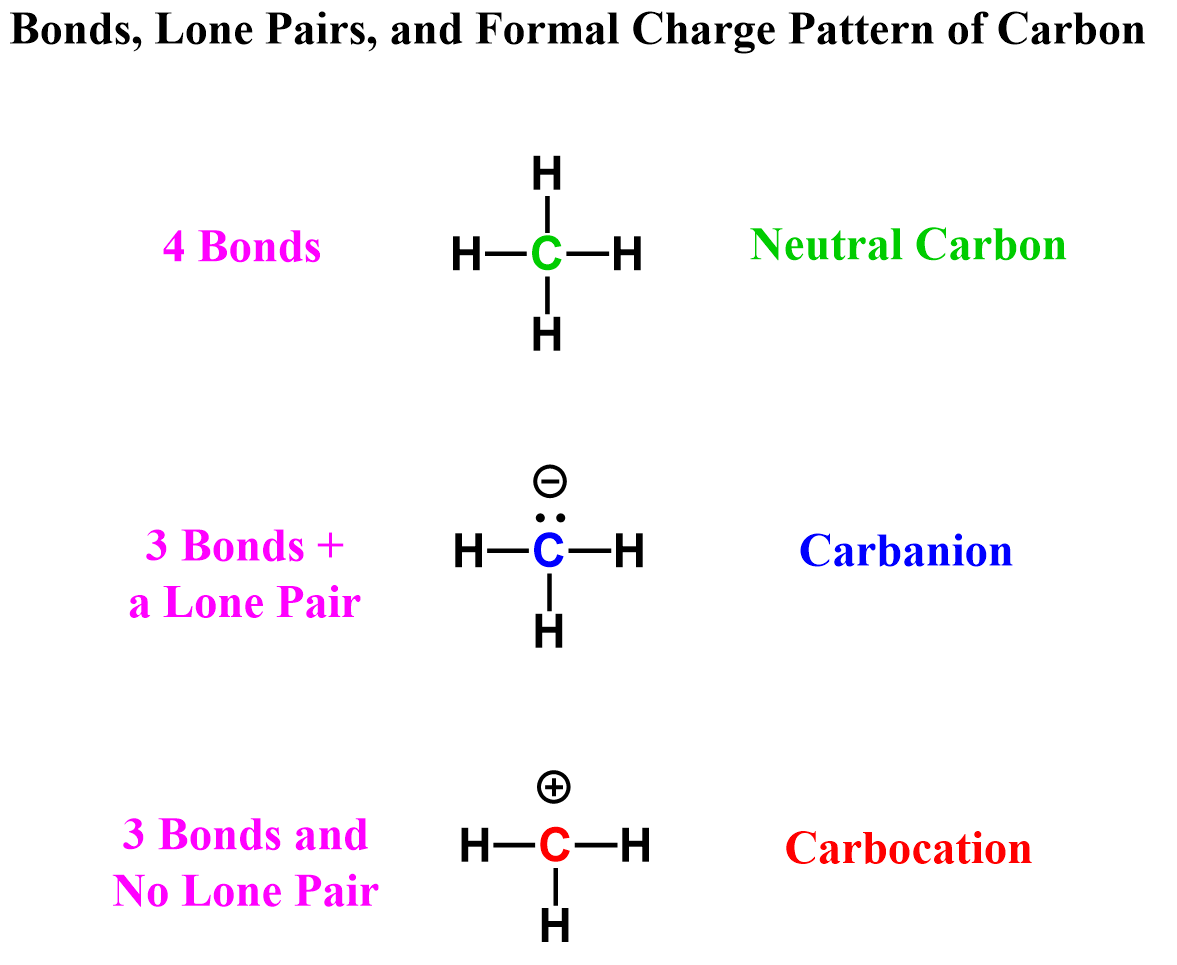
So, how do we know what formal charge is assigned to atoms? There are several formulas you can use to calculate the formal charges. They are all essentially the same, however, represented a little differently. For example, one of them is:
FC= V – (N + B)
Where:
V – number of valence electrons
N – number of nonbonding electrons
B – number of bonds
Recall from general chemistry that valence electrons match the periodic group
Valence Electrons (V)
Recall from general chemistry that you can determine the number of valence electrons by looking at the periodic table – just match the atom’s group number in the main group elements:
- Carbon (Group 4) → 4 valence electrons
- Nitrogen (Group 5) → 5 valence electrons
- Oxygen (Group 6) → 6 valence electrons
- Halogens (Group 7) → 7 valence electrons
Non-Bonding Electrons (N)
These are the individual electrons found in lone pairs.
Each lone pair contains 2 electrons, so:
- 1 lone pair = 2 non-bonding electrons
- 2 lone pairs = 4 non-bonding electrons, etc.
Make sure you count electrons, not the pairs.
Number of Bonds (B)
Each bond represents a pair of shared electrons.
So, when counting bonds:
- A single bond = 1 bond
- A double bond = 2 bonds
- A triple bond = 3 bonds
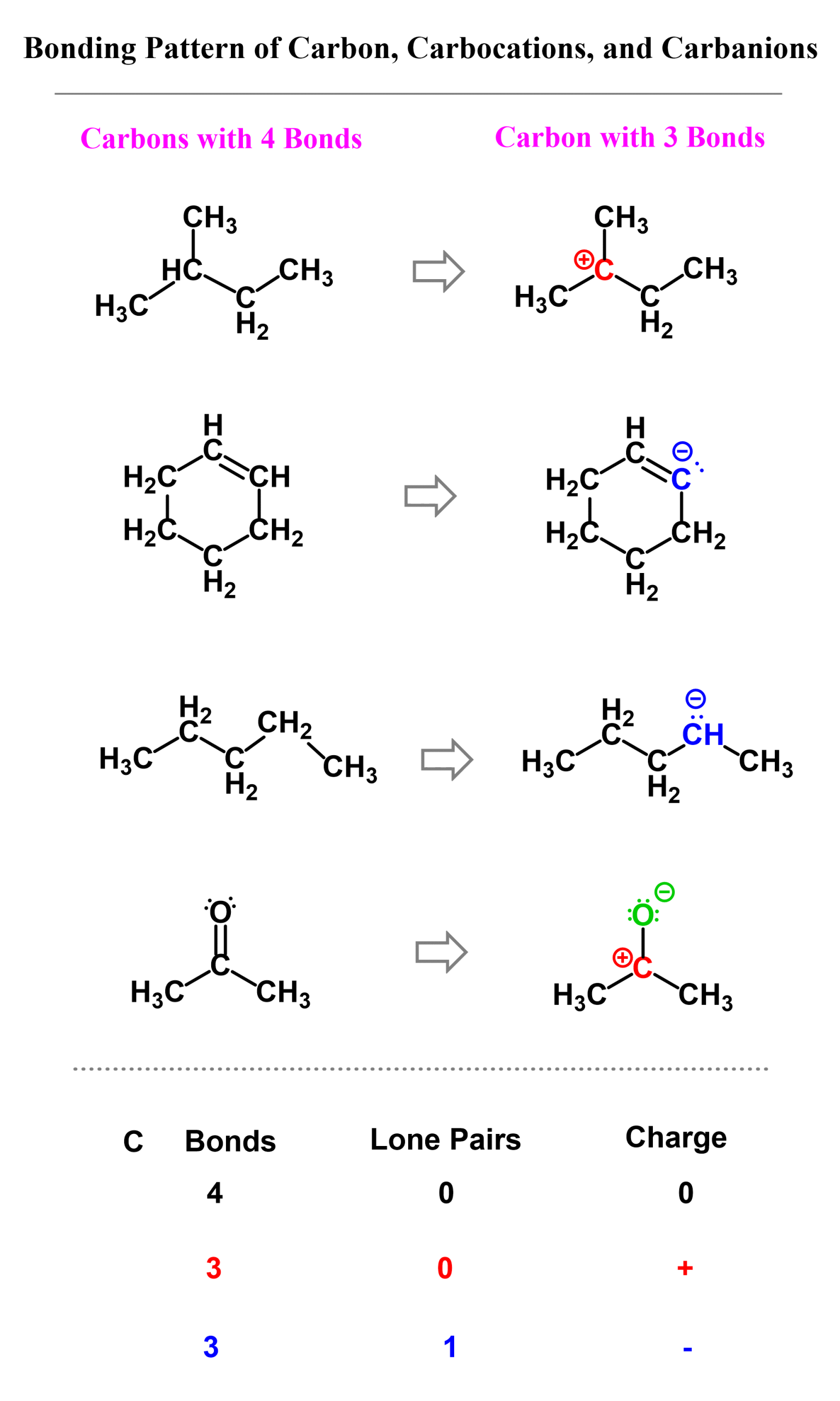
Here are some examples of using the formula for calculating the formal charge of carbon is neutral and ionic states where a formal charge is assigned:

Once again, your textbook might use a different formula for calculating the formal charge. They all mean the same thing. Just make sure you understand what each letter represents and how the number of bonds and lone pairs is counted.
Here are some more examples of carbon with and without formal charges.
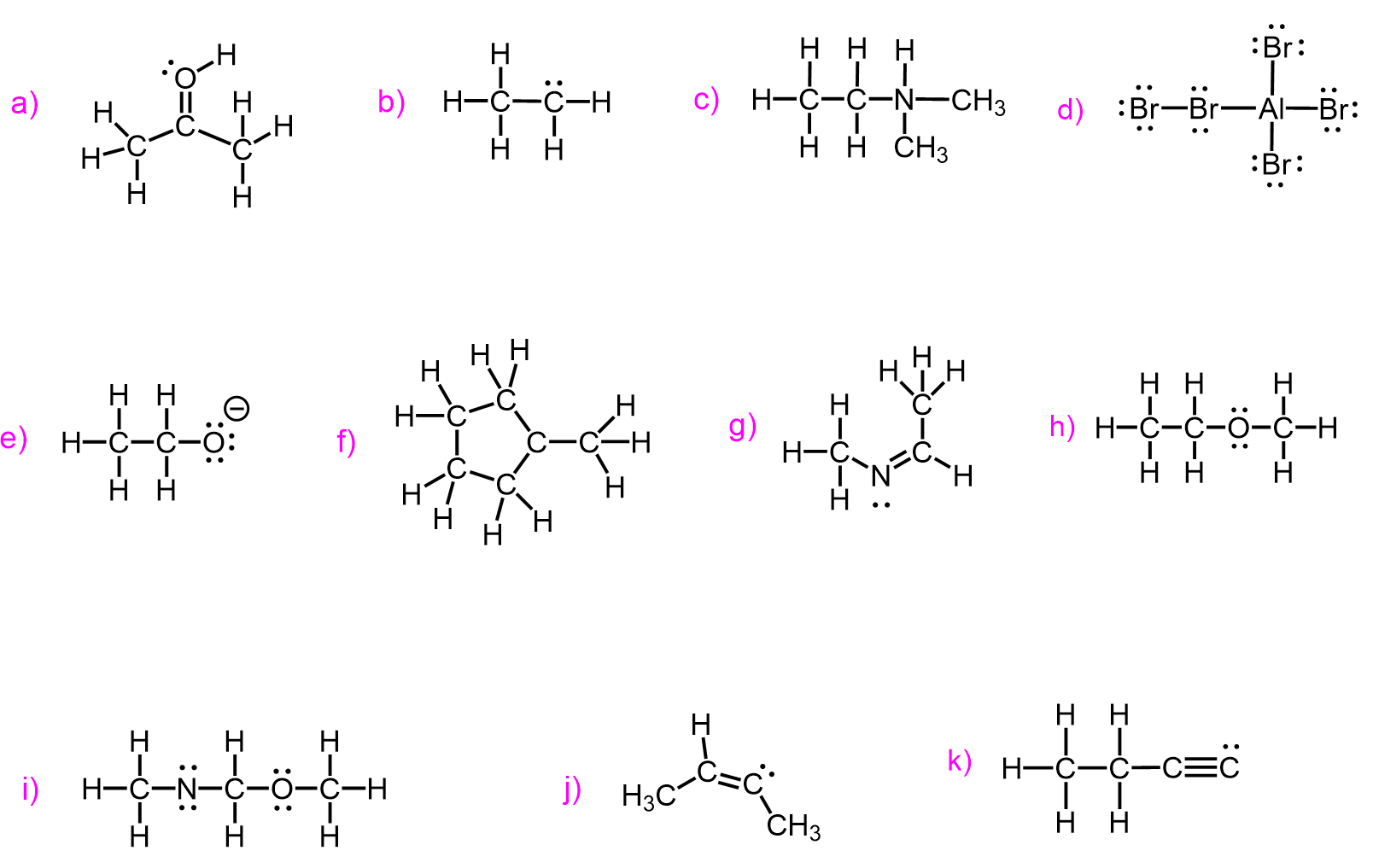
Formal Charges of Nitrogen, Oxygen, and Halogens
The same principles apply when calculating the formal charges of nitrogen, oxygen, halogens, and any other elements. Just like with carbon, you start by identifying the number of valence electrons based on the element’s position in the periodic table. Then, count the number of bonds and non-bonding electrons (lone pairs) around the atom in the structure. For example, nitrogen normally forms three bonds and has one lone pair, giving it a neutral formal charge. If it forms four bonds and no lone pairs, it carries a positive charge. Similarly, oxygen typically forms two bonds and has two lone pairs, so any deviation from this will result in a formal charge. Halogens usually form one bond and have three lone pairs.

Formal Charges Without Formula
As mentioned earlier, with time, understanding bonding patterns and formal charges will become natural, and you won’t always need to plug numbers into a formula to determine them. Each element in organic chemistry tends to “prefer” a certain number of bonds when it’s neutral: carbon likes four, nitrogen three, oxygen two, and halogens one. If an atom deviates from this typical bonding pattern, it usually carries a formal charge. For example, if nitrogen has four bonds instead of three, it’s likely positively charged. If oxygen has only one bond and three lone pairs, it likely has a negative charge. With practice, you’ll be able to spot these patterns at a glance and assign formal charges intuitively, without needing to count every electron.
Here is also a table that includes the bonding pattern of nitrogen, oxygen, and halogens in organic chemistry. As a side note, remember that hydrogen can only have one bond.

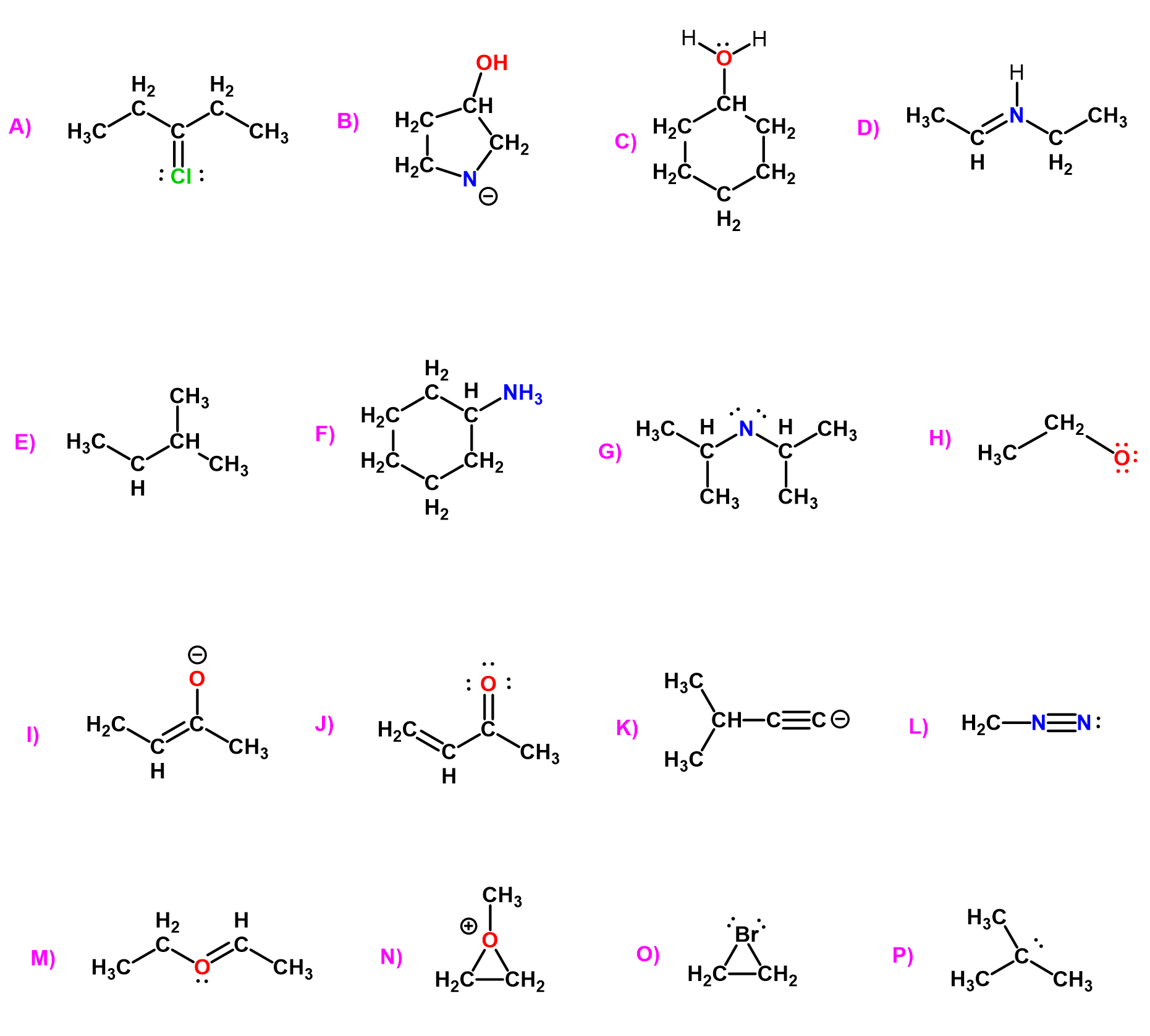
And of course, more examples of carbon and heteroatoms in organic molecules, both with and without formal charges. These patterns serve as a helpful reference when evaluating or correcting structures in organic molecules.
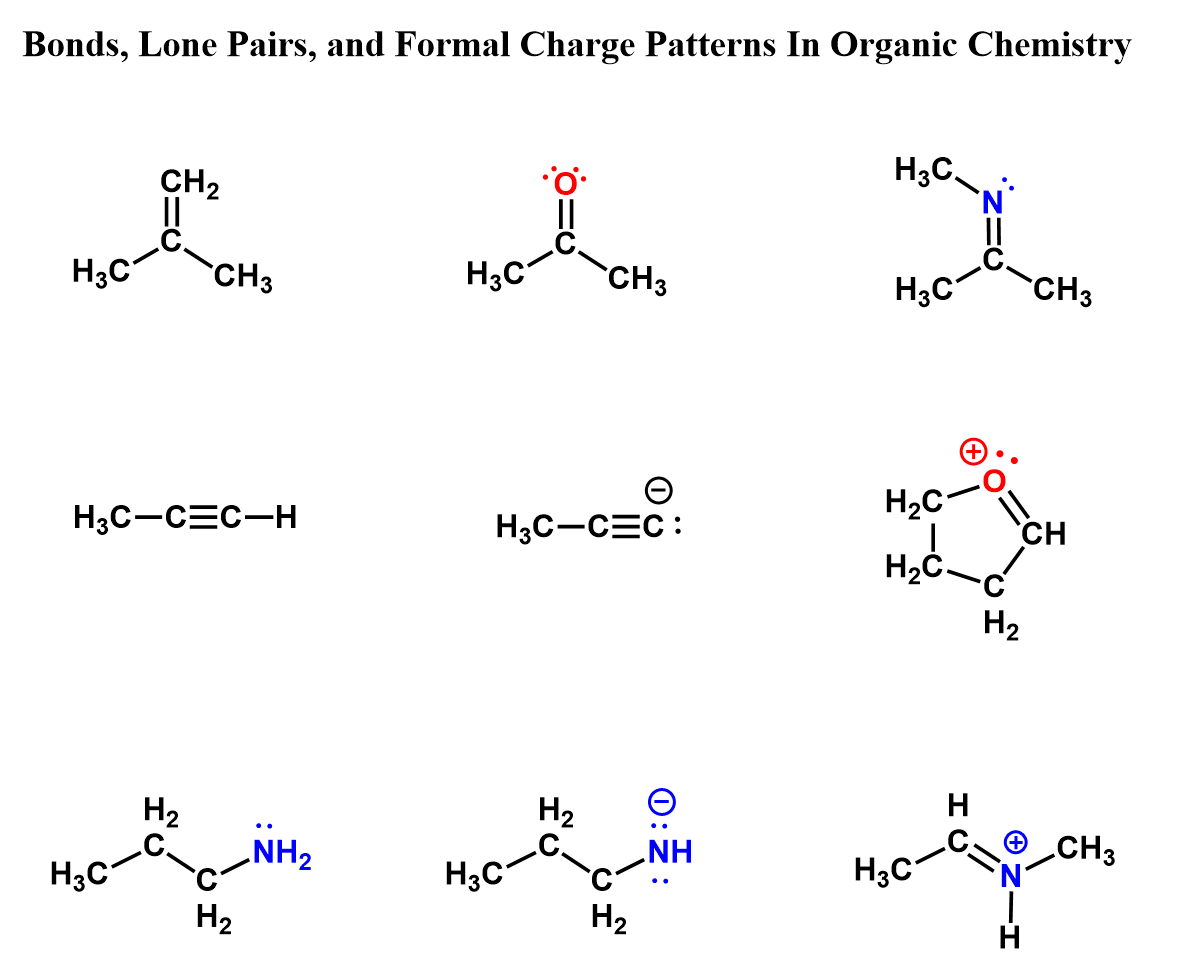
In the end, the best way to master bonding and formal charges is through practice, and in organic chemistry, that means drawing lots of structures. Take that pencil and a sheet of paper, make mistakes, and correct them. That’s how you learn. Don’t stress about perfection – just focus on building familiarity and confidence. It’s better to make those small errors now than on the exam.