We have learned that resonance structures are different Lewis structures of the same molecule. In other words, they are different ways of drawing the same species.
So, what does “different” refer to if they represent the same species? The term “different” refers to the electron distribution, such as the lone pairs and π bonds in the molecule, and by “the same,” we are referring to the positions of the atoms.

A common question that arises here is: Are the resonance structures equal if they represent the same molecule?
The answer is: No, they are not, because some of them resemble the actual structure more than others.
A quick reminder again: resonance structures are not real as such. Just like a hybrid fruit does not flip back and forth between two fruits, a molecule doesn’t interchange between resonance forms. However, the hybrid fruit may look more like one parent than the other. For example, we can mix pomelo and mandarin to get a sweet orange, which is the hybrid fruit – the actual representation of the molecule:
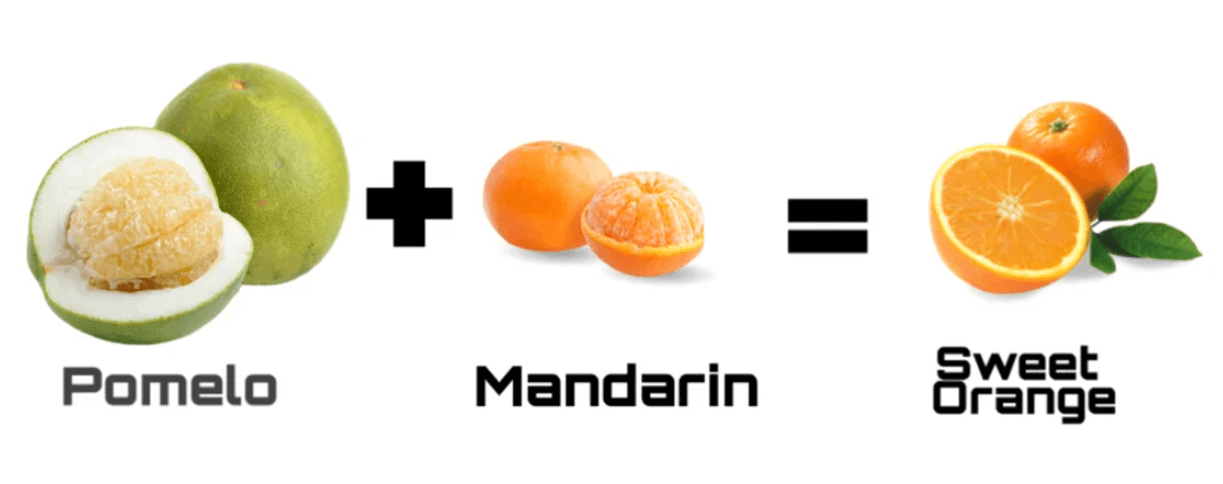
The sweet orange looks more like a mandarin than a pomelo, but that does not mean it exists as a mandarin most of the time. However, if someone asks you, “Oh, you bought some sweet oranges, what do they look like?”, you are going to say they look like mandarins. So, chemistry-wise, the term is “the mandarin is the major resonance contributor, and the pomelo is the minor resonance contributor.”
How Do We Identify the Major and Minor Resonance Contributors?
From the fruit analogy, your job here is to determine if it looks more like a mandarin or a pomelo. Which is to find the most stable resonance structure, as that’s the closest to what the molecule looks like.
With mandarins in mind, let’s look at some chemical structures.
What are the major and minor resonance contributors of this compound?

The terms “major” and “minor” contributors may sound confusing, but what we are doing is essentially determining the most stable resonance form, and then identifying the others that are still valid but not as stable as the hybrid form.
What factors affect the stability of resonance structures?
There are a few key parameters you need to remember when assessing the stability of resonance forms:
1) Neutral Molecules Are Preferred
- If charges are inevitable, place the negative charge on more electronegative atoms, and the positive charge on less electronegative ones. Avoid placing like charges on adjacent atoms.
2) Having Complete Octets Is Preferred
In some specific cases, other factors such as the degree of substitution of a carbocation, aromatic stability, etc., may matter, but for now, let’s focus on the main factors.
From Rule 1, we can identify that structure B is the major resonance contributor, as it has no charges. All the other forms have formal charges, so we need to check the octets of the atoms.
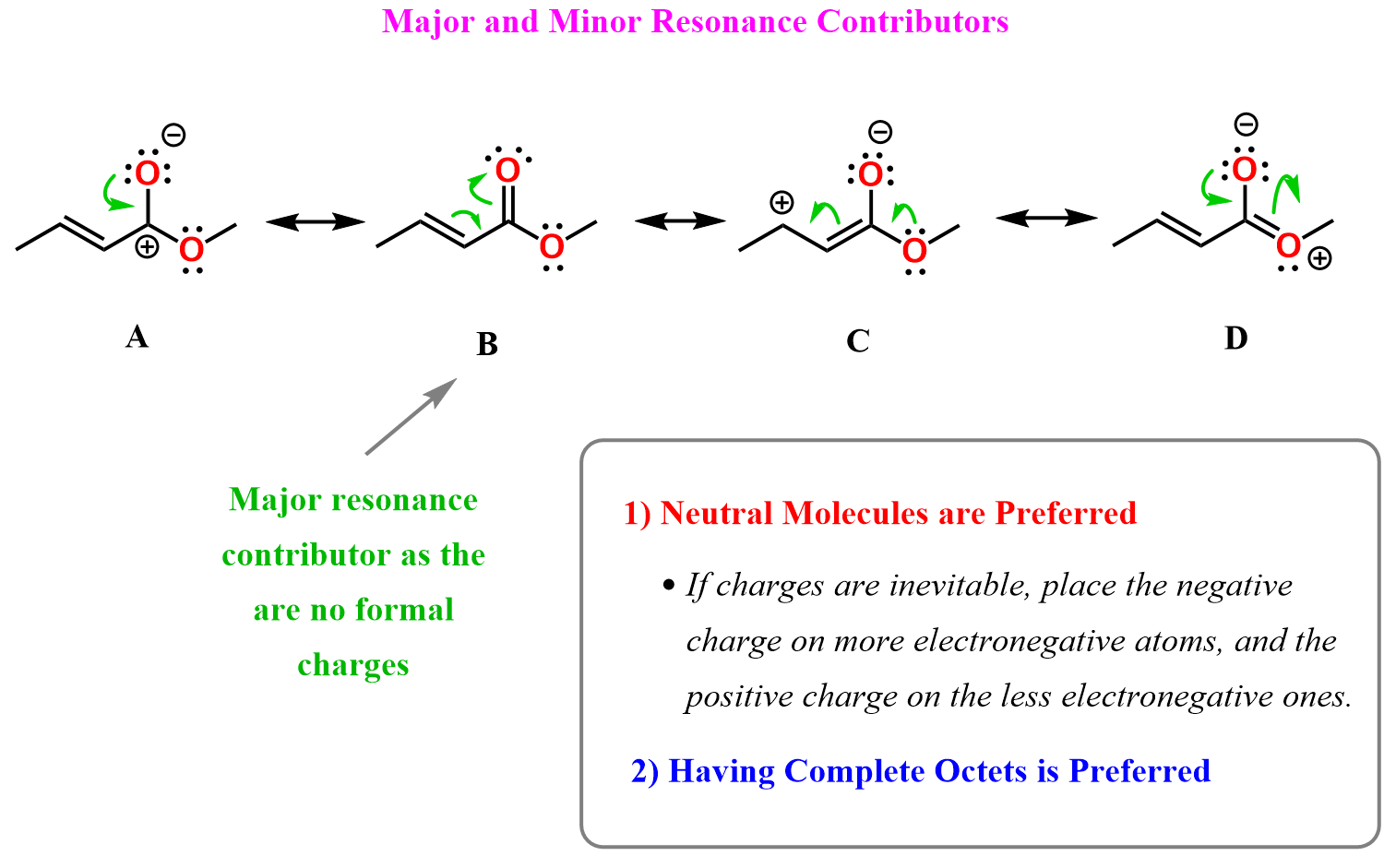
Aside from B, the only resonance form where all atoms have octets is D, so that would be the second most stable contributor.
Notice that in D, the positive charge is on oxygen, whereas in C and A, it’s on carbon. However, the octet rule is a greater factor, and we generally give preference to structures where all atoms have complete octets.
A and C would have about equal contributions to the resonance hybrid, as both have a negative charge on oxygen and a positive charge on carbon.
Stability of Negative Charges
The negative charge is a high density of electrons, so in order for the charge to be better-stabilized, these electrons need to be on a more electronegative atom.

This observation works best only when the two atoms bearing the formal charge are in the same row of the periodic table, since they have comparable atomic sizes.
However, as we go down the group, the atomic sizes increase, which helps to handle the negative charge more efficiently because the charge density decreases with large volume/surface.
Therefore, if you are comparing elements from different rows in the periodic table, choose the one where the charge is on the bigger atom as the major resonance structure:

If the negative charge is on the same atom in both resonance structures, then look for other factors that can stabilize it. For example, if the charge is next to an electronegative atom, it helps to stabilize it as well (inductive effect):

Check the stability of the conjugate base in an acid-base reaction for more details about stabilizing the negative charge.
Stability of Positive Charges
The stability trends for a positive charge are, as expected, opposite to the ones for the negative charge. The positive charge is a center of electron deficiency; therefore, to stabilize it, we need electron-donating groups. Alkyl groups (CH3-, CH3CH2-, etc.), being electron-donating, stabilize the positive charge on a carbon atom (carbocation):

1o, 2o, and 3o stand for primary, secondary, and tertiary, respectively. This shows the number of carbons (alkyl groups) connected to the positively charged carbon. The more alkyl groups, the more stable the carbocation.

For the same reason, putting the positive charge next to an electron-withdrawing group makes it less stable:

Notice that in none of the examples, we had a structure with more than one formal charge. So, remember that any resonance form with an atom bearing a +2 or −2 charge is very unstable and cannot be a significant contributor to the resonance hybrid.
Other Factors Contributing to Resonance Stability
If you’re in the chapter on resonance, you’re probably not yet discussing conjugated and aromatic systems, but these are important too.
Conjugated systems have alternating double and single bonds and are associated with extra stability.

An important class of conjugated systems is aromatic compounds. Think of these as rings with alternating double bonds, which leads to a particularly stabilizing electronic configuration.
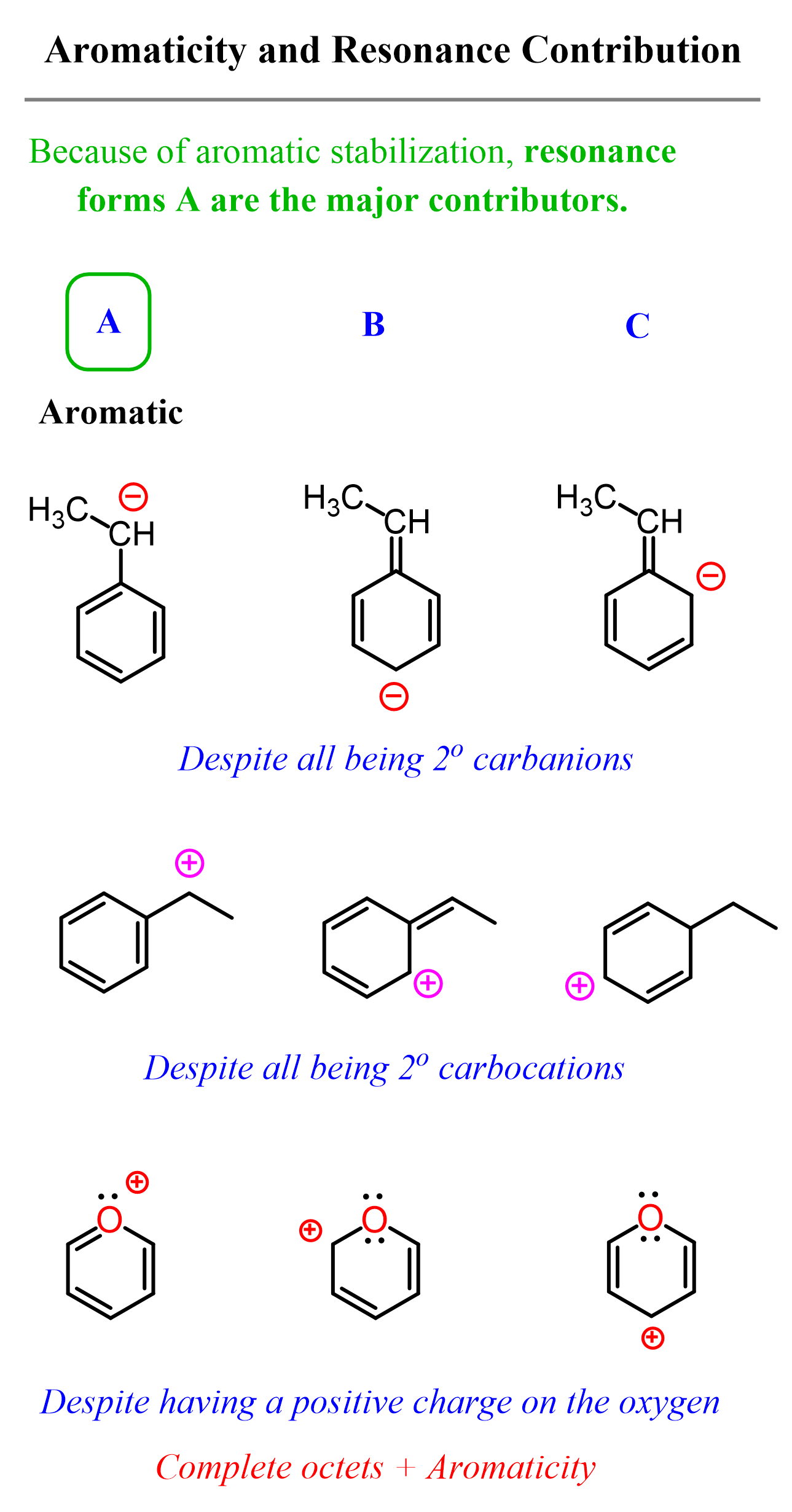
So, whenever possible, give preference to structures that maintain the aromatic structure.
For example, in all the examples shown below, resonance form A is the major contributor because of the aromatic stability:
What Are Significant Resonance Contributors?
In simple words, which is what I try to stick to, the term “significant resonance contributor” refers to all the resonance forms that do not break any rules.
We are not talking about exceeding an octet on carbon or breaking single bonds. Those are fundamental rules we learn to draw resonance structures. They are not there to check a structure that is not valid or not a resonance. Instead, we’re talking about structures that are technically not incorrect, but don’t meaningfully contribute to the hybrid.
For example, if you go from structure B to C, you are pushing electrons from a carbon to an oxygen, which aligns with basic principles that we know even from General Chemistry 1 – electrons tend to shift to more electronegative atoms.

If we pushed the electrons of a C=O double bond toward the carbon instead of the oxygen, that would create an insignificant resonance structure. Compare these two patterns below:
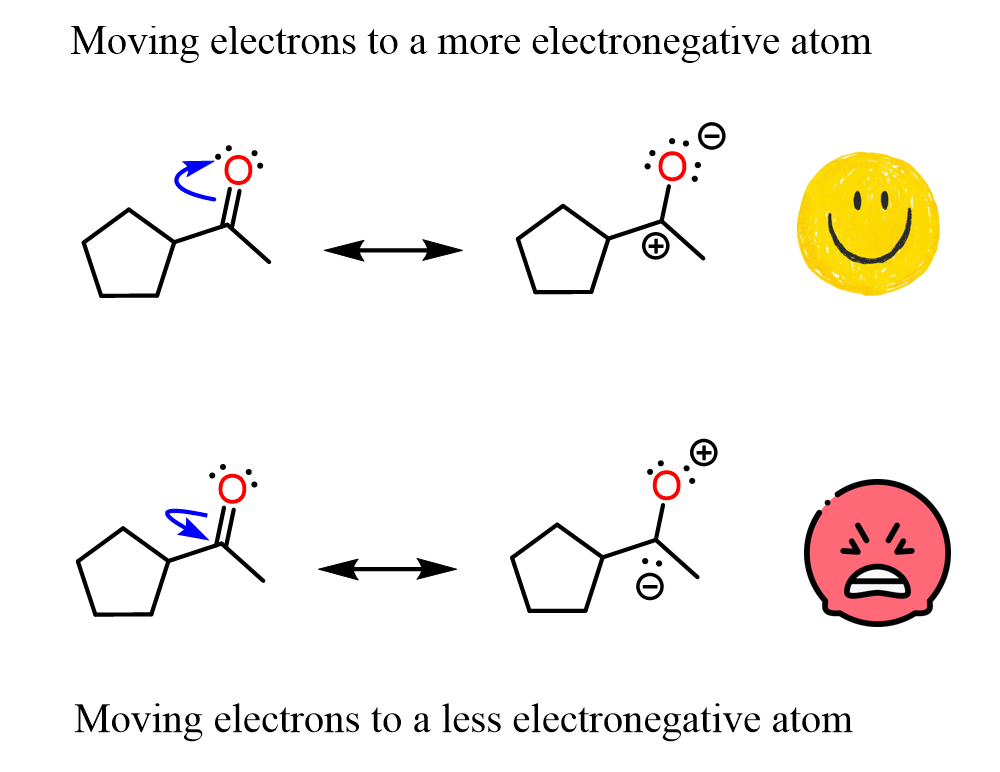
Once again, structurally, there is nothing “wrong” with the second representation, so we call these insignificant, rather than incorrect, resonance structures. Yes, the oxygen lacks an octet, and that is pretty bad, but it’s not like it has five bonds. I hope that makes sense.
Let’s summarize what we learned about major and minor resonance contributors
Resonance structures are different Lewis structures that represent the same molecule. They differ only in the position of electrons, not the atoms. These forms help us understand how electrons are distributed across a molecule and give us a better picture of the true structure, which we call the resonance hybrid.
However, not all resonance structures are equal. Some are more stable and contribute more to the hybrid. These are called major contributors. Others are less stable but still follow the rules. These are minor contributors.
To identify the most stable resonance structure, keep these key rules in mind:
1) Neutral structures are more stable than charged ones.
- If charges are inevitable, place negative charges on more electronegative atoms, and positive charges on less electronegative atoms.
- If you are comparing atoms from the same group in the periodic table, larger atoms handle negative charges better. For example, S– > O–.
2) Complete octets are preferred.
3) Conjugation and aromaticity add extra stability and should be favored when possible
You will also see a lot of the terms “Significant and Insignificant Resonance Structures”.
- Significant resonance structures follow the rules of drawing resonance forms.
- Insignificant resonance structures may not be technically incorrect, but for simplicity, treat them as incorrect resonance structures.
Check this 60-question, Multiple-Choice Quiz with a 2-hour Video Solution covering Lewis Structures, Resonance structures, Localized and Delocalized Lone Pairs, Bond-line structures, Functional Groups, Formal Charges, Curved Arrows, and Constitutional Isomers.
Molecular Representations Quiz




