In the previous post, we learned that alkyl substituents are the smaller fragments on the parent chain. To name them, we change the suffix (ending) of the corresponding alkane from –ane to –yl. For example:

For the methyl and ethyl substituents, we can only have one connection point to the parent chain because methane has only one carbon, and ethane is symmetrical.
For propane, we can have the connection point either at the terminal carbons or the middle carbon, which means there can be two propyl substituents: n-propyl (or just propyl) and iso-propyl:
Now, for four carbons, there are more variations of how they are connected and which of them is going to be connected to the parent chain. We have n-butyl (butyl), sec–butyl, tert–butyl, and iso–butyl.
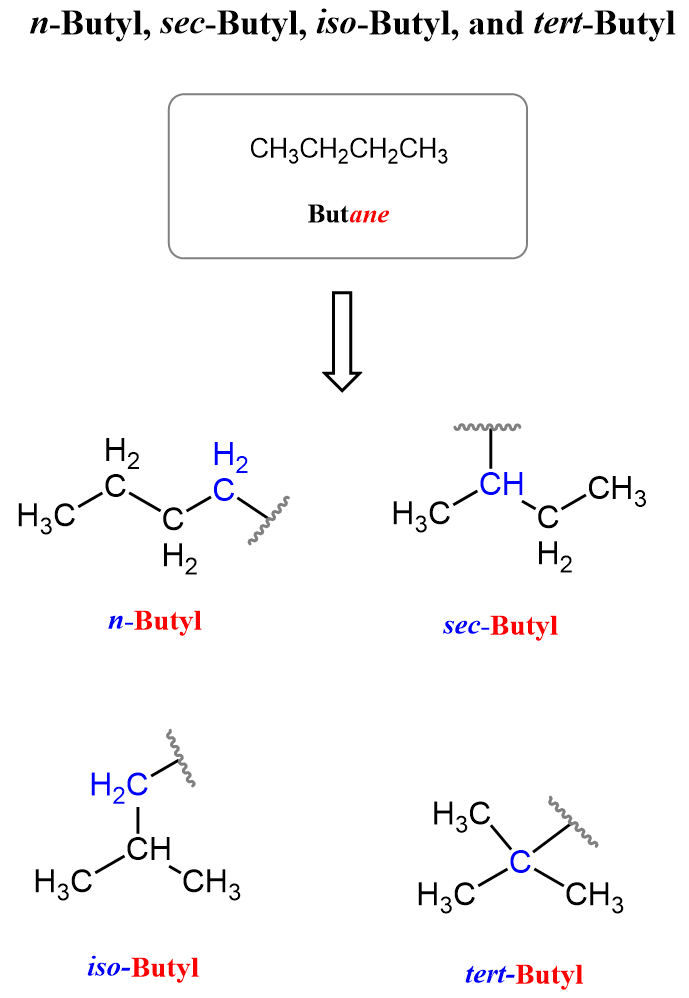
So, what do the terms “n-”, “sec-”, and “tert-” mean?
“n” stands for normal, which implies a straight chain of four carbon atoms that are connected to the parent chain via the terminal carbon atom.
“sec” stands for secondary, which means it is connected to the parent chain via a secondary carbon atom – that is, carbon 2 in butane.
Therefore, “tert” indicates that the alkyl chain is connected to the parent chain via a tertiary carbon atom:

The green circles are to highlight the number of carbons connected to the one that is connected to the parent chain. For example, in sec-butyl, there are two carbons connected to the bridging carbon, which means the latter is a secondary carbon. Notice that we are talking about when the sec-butyl group is by itself. When it is connected to the parent chain, that carbon is already tertiary.
These names are used not only for the alkyl groups connected to a parent chain, but also when another atom or a group is connected to them. That is why we do not count the carbon atom of the parent chain.
For example, although not the official IUPAC names, we have tert-butyl chloride, n-butyllithium, tert-butyllithium, etc.

Butyl Groups and IUPAC Nomenclature
Sec-butyl, iso-butyl, and tert-butyl are not the official (accepted/preferred) IUPAC names of these substituents. This, of course, does not mean that they are wrong; it is just that if we (your professor) want to follow the rules of IUPAC nomenclature, we need to name them accordingly. “Accordingly”, means we recognize them as complex substituents and name them as if they are a small molecule on their own attached to the parent chain.
We have a separate post on naming complex substituents, so feel free to check that out here, but in short, we identify a parent chain and substituents within these alkyl groups, and add that name to the actual parent chain by changing the suffix from -ane to -yl:
Therefore, for isobutyl we have 2-methylpropyl, for sec-butyl it is butan-2-yl (also known as 1-methylpropyl), and for tert-butyl it is 1,1-dimethylethyl.
Here is an example of how a molecule with a sec-butyl substituent is named:

In the second option, sec-butyl is named as a complex substituent by identifying the parent chain and substituents on it:
-
The parent chain within the substituent is a three-carbon chain (propane),
-
There is a methyl group attached to carbon 1 of this chain,
-
And the point of attachment to the main molecule is also at carbon 1.
So, we are taking 1-methylpropane, dropping the -ane and replacing it with -yl, which gives the IUPAC name 1-methylpropyl.
This is the preferred IUPAC name for what we commonly call sec-butyl.
Once again, both are correct, but according to the latest IUPAC rules, we should not say sec-butyl; it should be 1-methylpropyl. Most textbooks and professors would be fine with either option, but again, check with your professor, as ultimately it is their preference that matters for your course and exams.
Check the article “Naming Alkanes with Practice Problems” for more such examples and practice problems.
Summary of n-, sec-, iso-, and tert-Butyl Groups
All the butyl groups with different prefixes are used to describe a carbon chain fragment with four carbon atoms, but they differ based on how the atoms are arranged and which carbon is connected to the parent chain, another group, or an atom.
These prefixes are important because simply saying “butyl” isn’t specific enough: there are multiple ways to arrange four carbon atoms, and the point of attachment to the parent chain can vary. These differences affect the shape, branching, and reactivity of the molecule.
-
n-Butyl (“normal”) is the straight chain of four carbons connected via the terminal carbon atom.
-
sec-Butyl (“secondary”) is the straight chain of four carbons connected via the secondary (connected to two carbon atoms) carbon atom.
-
iso-Butyl is a branched chain of four carbons with a methyl group on carbon 2, connected via a terminal carbon atom.
-
tert-Butyl (“tertiary”) is a branched chain where three methyl groups are attached to a central carbon atom, which is connected to the parent chain.
Keep in mind that these are not the preferred IUPAC names, so always clarify with your instructor how they should be named for your organic chemistry exam.
Check Also
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Newman Projection of Chair Conformation
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
