Doing practice problems is the only way to master organic chemistry! At Chemistry Steps, you can find all the topics of Organic 1 and 2 and their associated practice problems. There are more than 2000 practice questions, and you can find them after each article listed below.
Here are some examples from the topics shown below.
Note: These are sample practice problems for the new users. Please go to the topics, and you can practice problems after the articles, or in a separate post.
Registered members can access all the quizzes and their solutions too.
Organic Chemistry 1 Practice Problems
Structure and Bonding Practice Problems
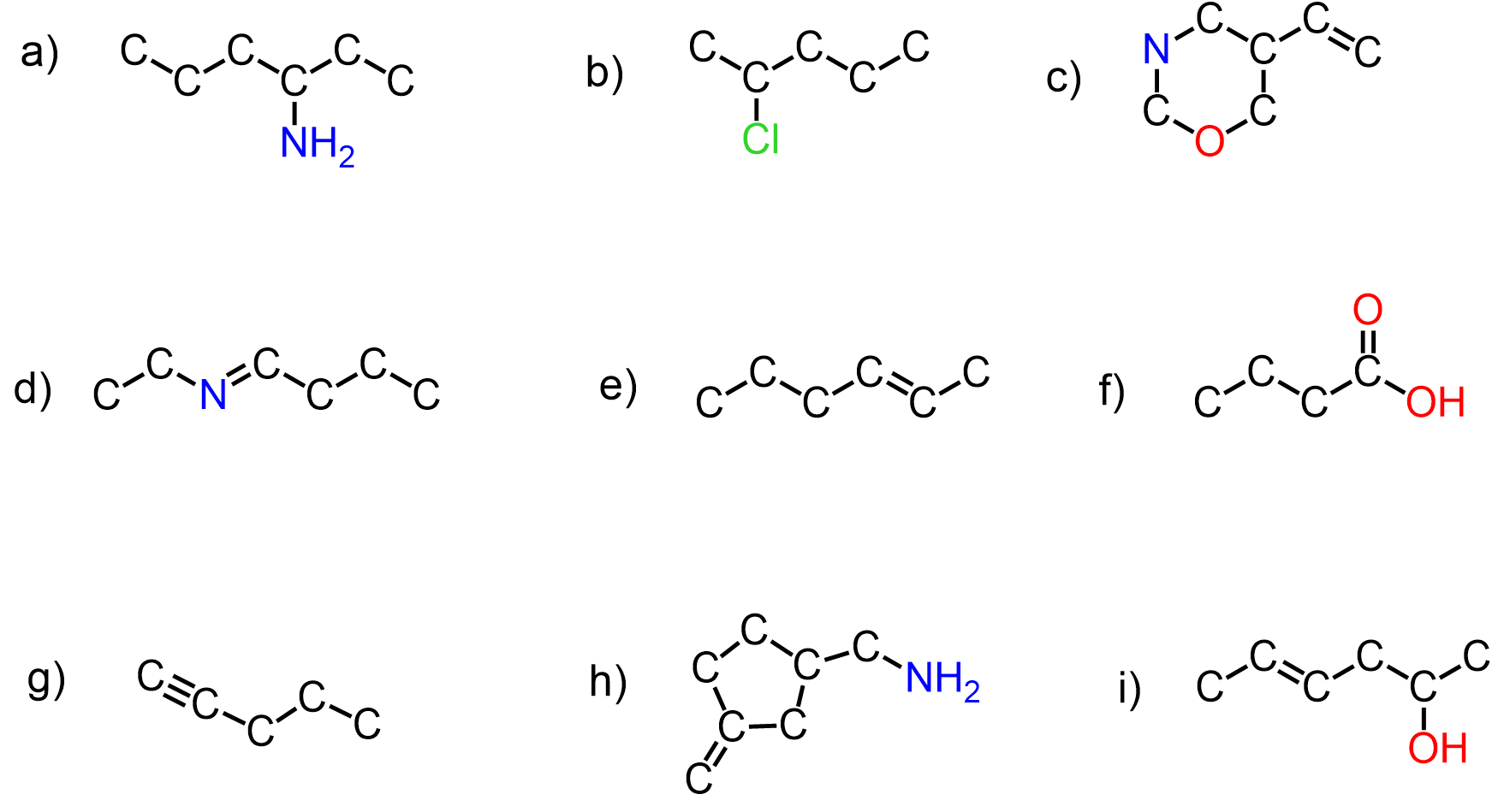
Convert the following condensed structures into Bond-line structures:
a) CH3CONHCH2OCH3
b) O(CH2)3CHCH3
c) CH3CHCHN(CH3)CH2CH3
d) (CH3)3CCH2CH2COOCH2CH3
e) CH3CH2CCCH2CO(CH2)2N(CH3)2
f) CH3CH2C(CH3)CHCH(CH2)4
g) (CH3)2CCHCH2OCH(CH3)CH2CN
h) CH3CH2CHClCHBrCH2CONHCH(CH3)2
i) (CH3)2CHCCCH2OCH(CH2Br)CH2CH3
j) CH3NHCHCCH3CHClCON(C2H5)2
Molecular Representations
Practice Problems
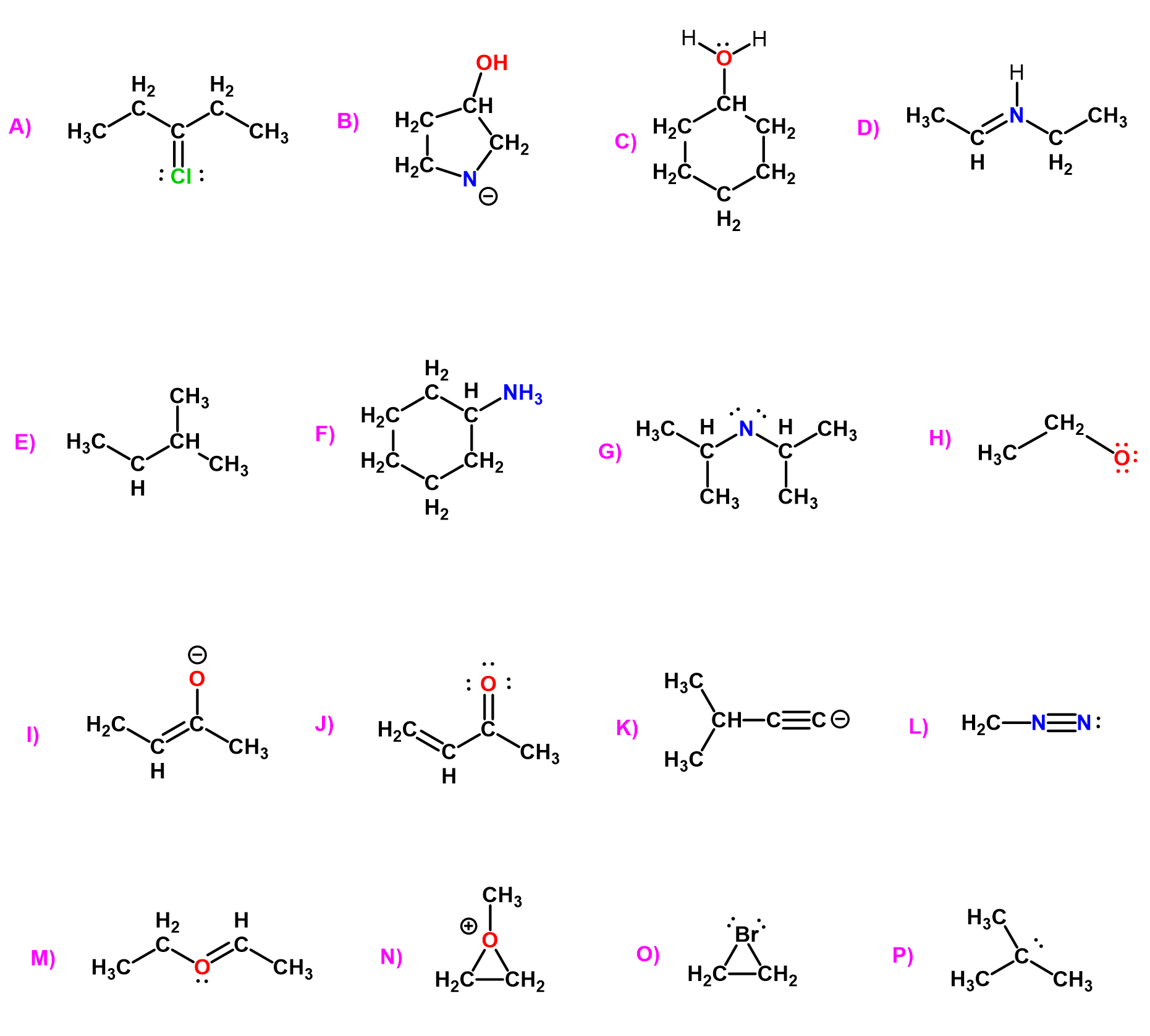
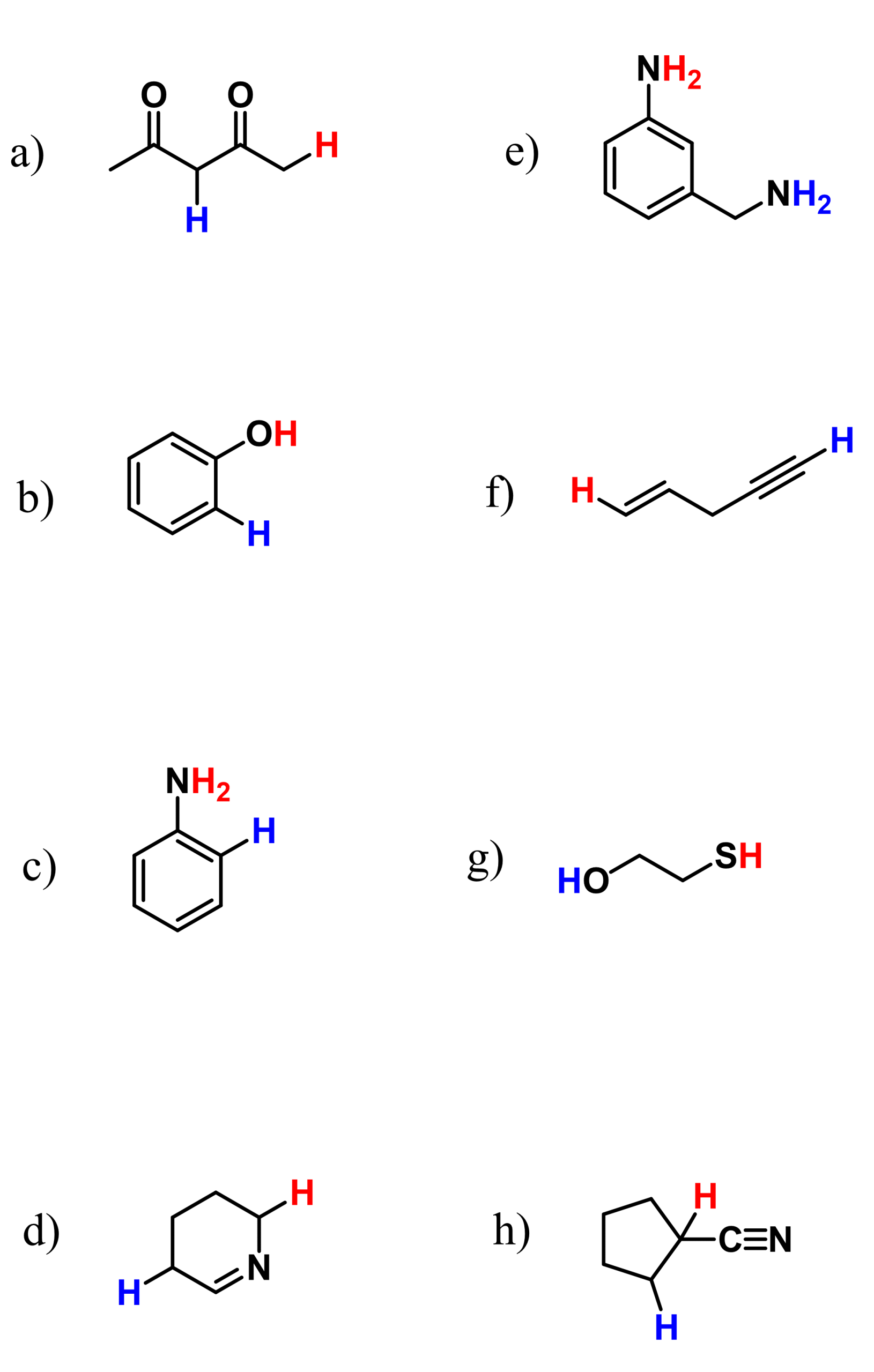
Identify the delocalized lone pairs of electrons for each of the following compounds and draw at least one resonance structure using the delocalized lone pairs.

Acids and Bases Practice Problems
Using the pKa table, determine a suitable reagent to deprotonate the following compounds. Each reagent can only be used once. Indicate the pKa values and write the second product as well.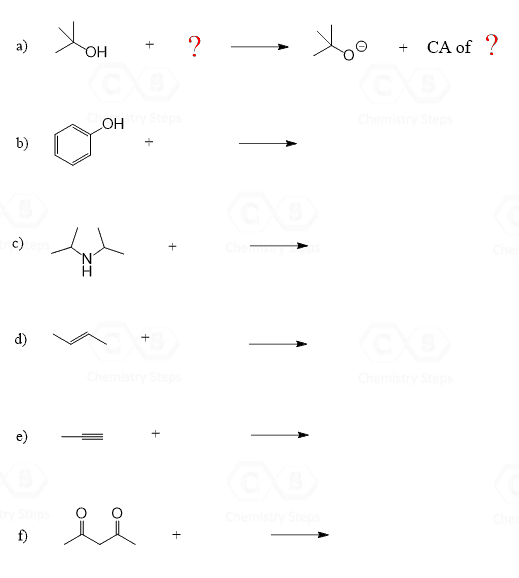
Alkanes and Cycloalkanes Practice Problems
Draw both chair conformation (ring-flip) and use the table to calculate the relative energy cost associated with each group in the axial position to determine the more stable chair conformation of each of the following compounds:

Stereochemistry Practice Problems
Determine if each of the following alkenes has an E or Z configuration:

Determine the relationship in each of the following pairs. Do the drawings represent constitutional isomers or stereoisomers, or are they just different ways of drawing the same compound? If they are stereoisomers, are they enantiomers or diastereomers?
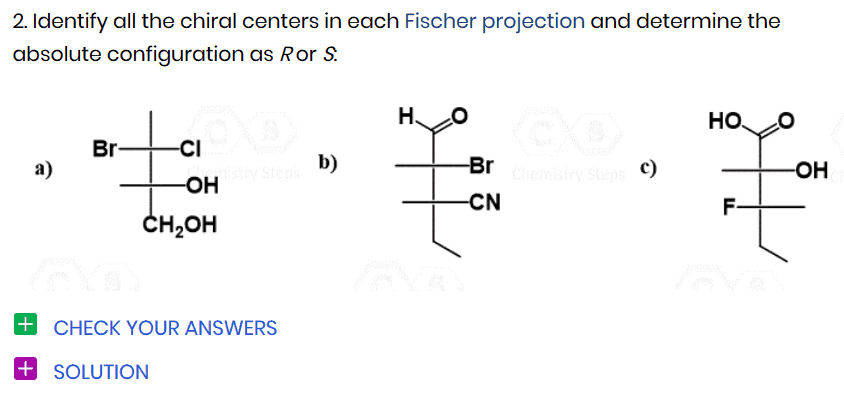
Explain your answer by converting the drawings into the same representation, i.e., if you are comparing a Newman projection to a Fischer projection, you need to convert both into either a Newman or Fischer projection. Assign all the absolute configurations as R or S if you hesitate.

Identify all the chiral centers in each molecule and determine the absolute configuration as R or S:

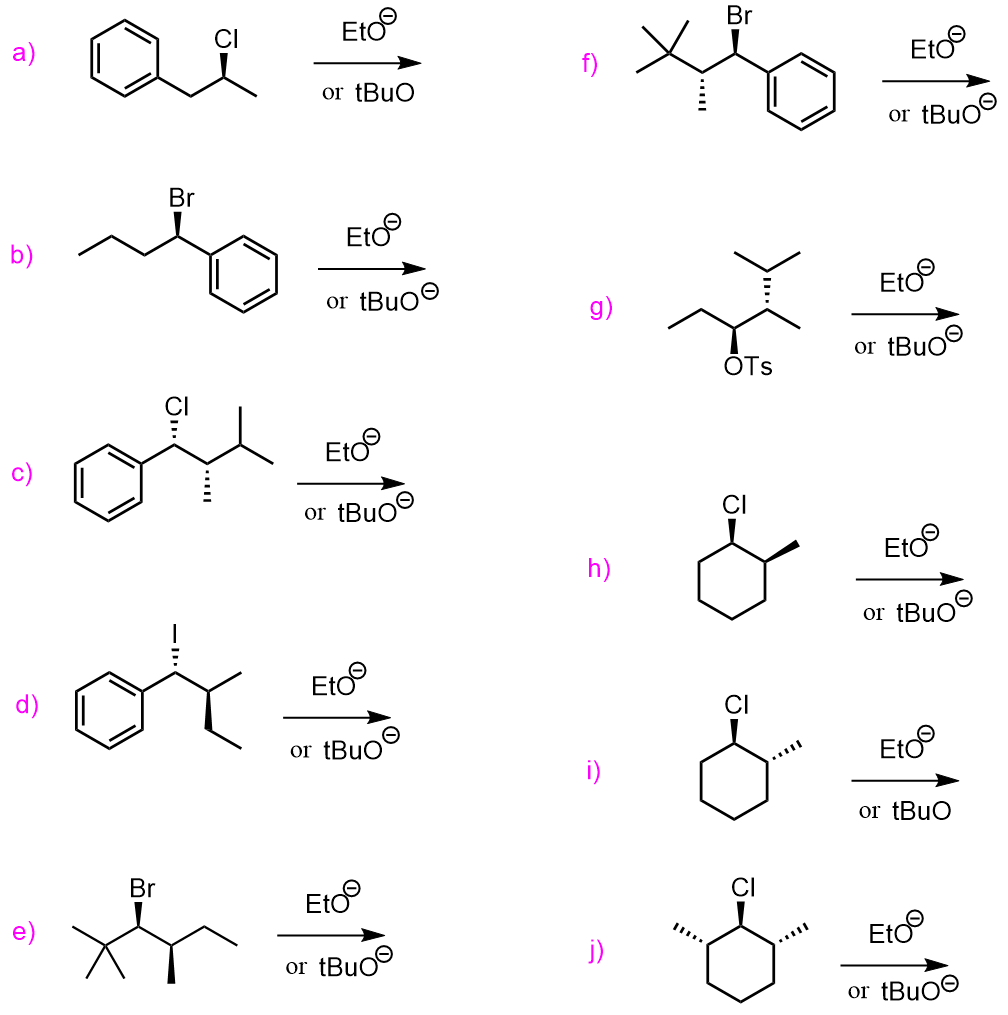
Nucleophilic Substitution and Elimination Reactions
Practice Problems
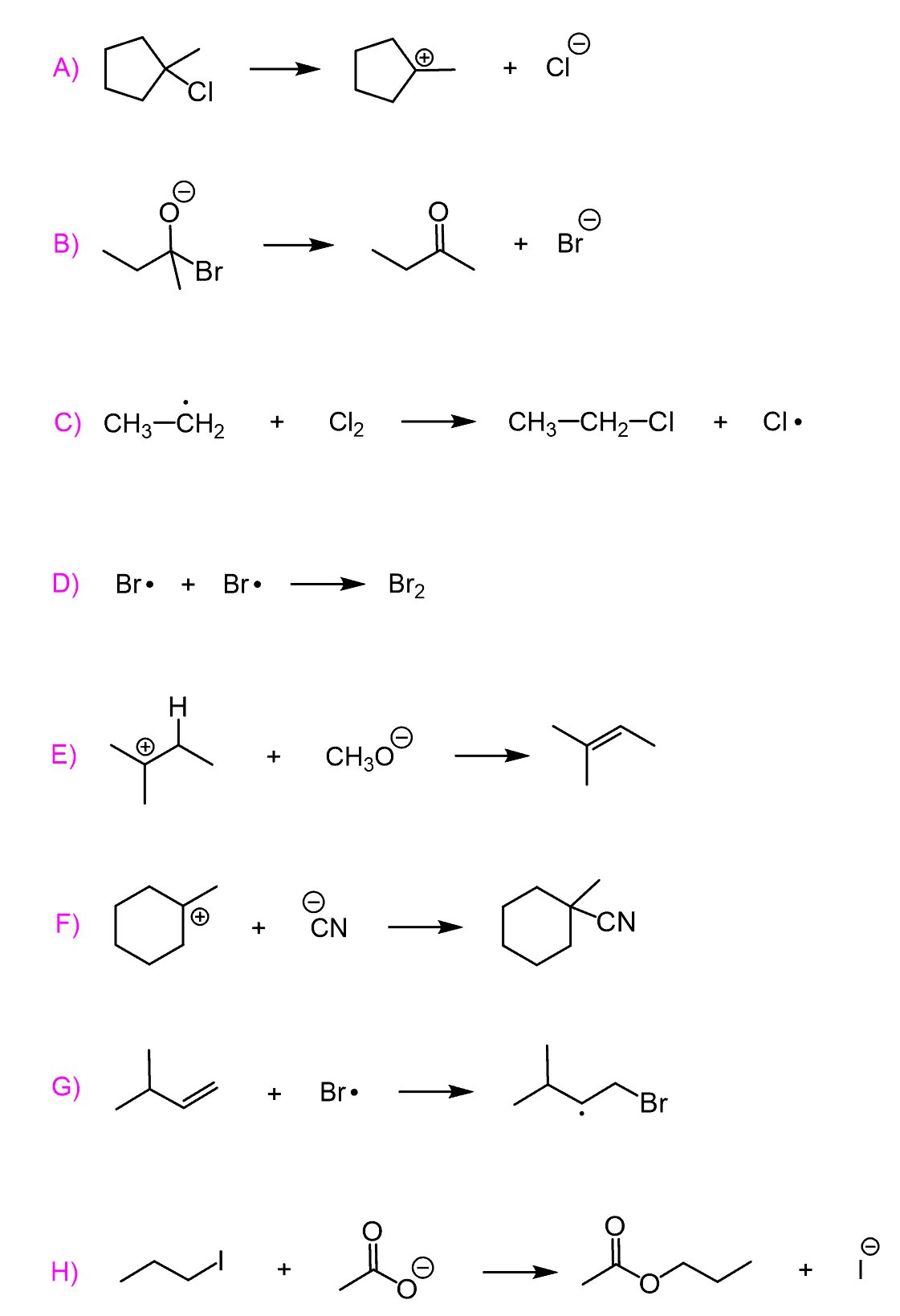
Predict the mechanism as SN1, SN2, E1, or E2 and draw the major organic product formed in each reaction. Consider any regioselectivity and stereoselectivity where applicable:

Reactions of Alkenes Practice Problems
Identify the reagents for each of the following addition reaction to an alkene:

Predict the product(s) that are formed after each step for reactions A-G. In each case, consider the formation of any chiral center(s) and draw all expected stereoisomers.
This is a comprehensive problem that covers the following topics and will serve as a review of all of them:
Substitution and elimination reactions. Particularly, substitution and elimination reactions of alcohols, the regio– and stereochemistry of E2 reactions and E2 reaction of cyclohexanes.
Mesylation and tosylation in Substitution and elimination reactions.
Hydrohalogenation of alkenes according to the Markovnikov’s rule.
Radical hydrohalogenation of alkenes.
Hydroboration-Oxidation of Alkenes.
Halogenation of alkenes through halohydrin formation.
Syn and anti dihydroxilation of alkenes.
Elimination reactions: Zaitsev and Hoffman products.
Ozonolysis of Alkenes.

Attempt to solve the entire problem before accessing the answers!
The answers will give you the structure of the final product(s) only. Use this as a hint to determine the compounds formed after the first and second reactions.
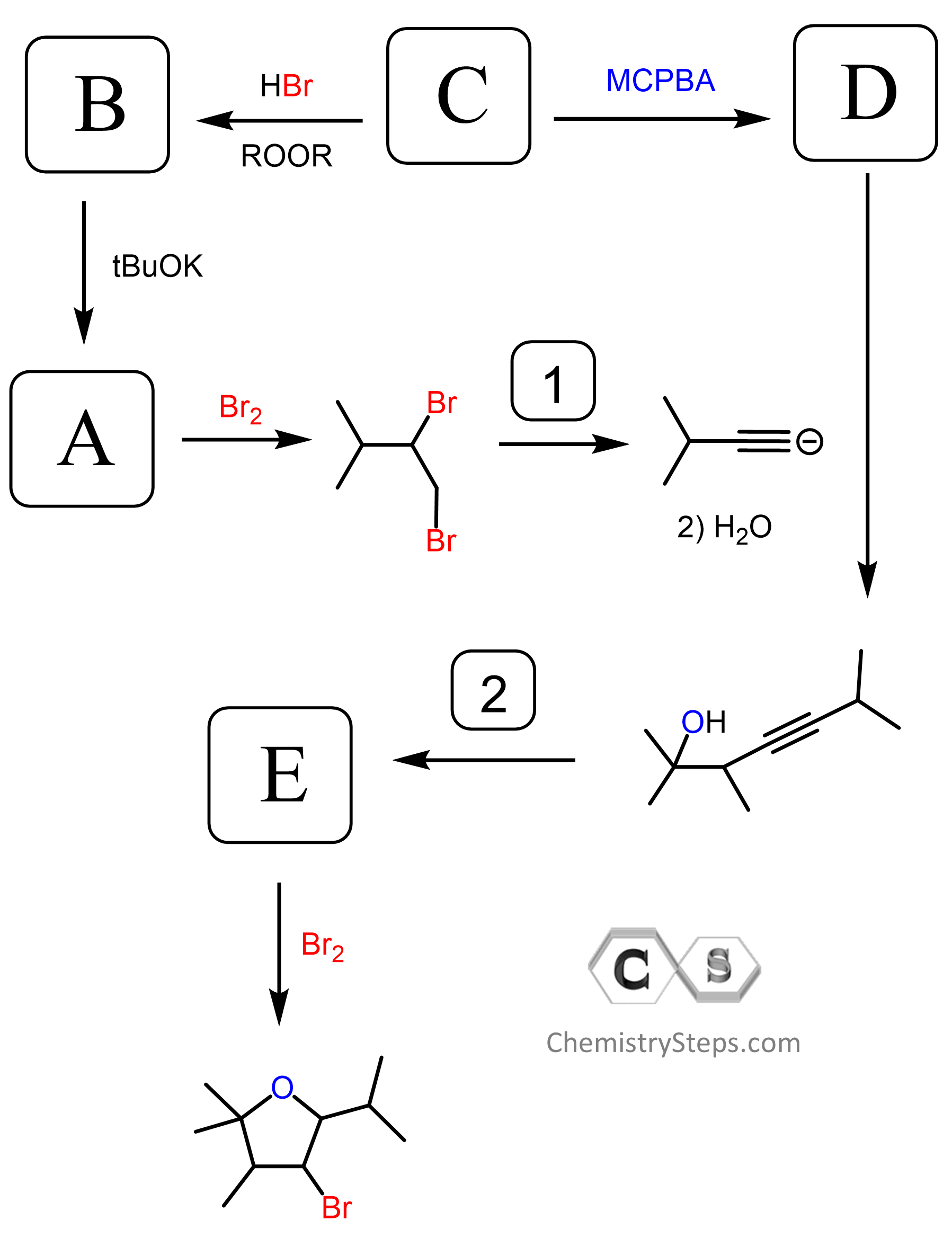
Reactions of Alkynes Practice Problems
Identify the reagents for each of the following reactions of internal and terminal alkynes:

On the following synthetic scheme, identify the reagents, in the correct order, that you would use to achieve the following synthetic transformations. Determine the structure of compounds A and B and the major organic products resulting from the alkyne.
Organic Chemistry 2 Practice Problems
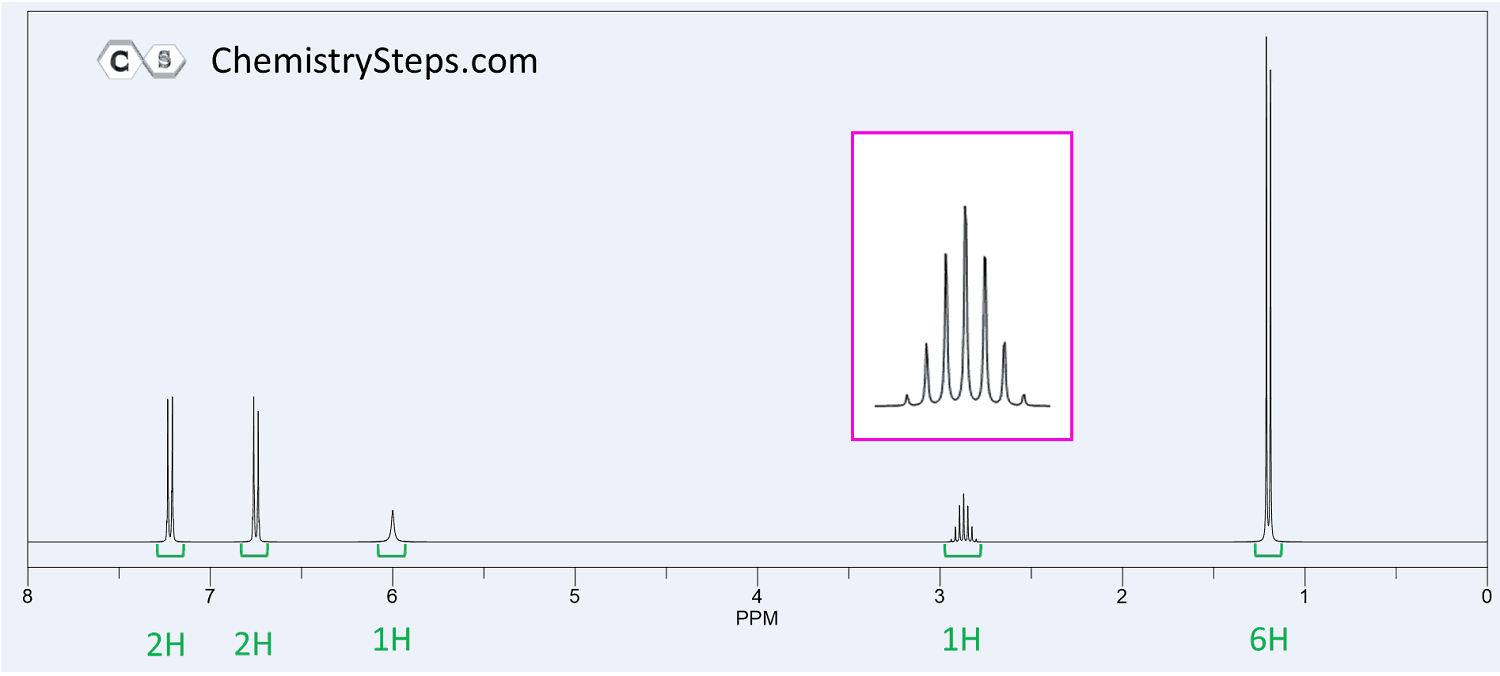
NMR Spectroscopy Practice Problems
The 1H NMR spectrum of compound X (C4H8O2) is shown below. It also shows a strong IR absorption band near 1730 cm−1. Propose a structure for X.

IR Spectroscopy
Label the functional groups and identify the correct compound based on the IR spectrum.

Radicals Practice Problems
Predict the products when each of the following compounds is treated with NBS under UV light:

Alcohols Practice Problems
Predict the major organic product(s) for the following Grignard reactions of a ketone, aldehyde, ester, carbon dioxide and an epoxide:

Predict the major organic product when the following alcohol is treated with each oxidizing agent:

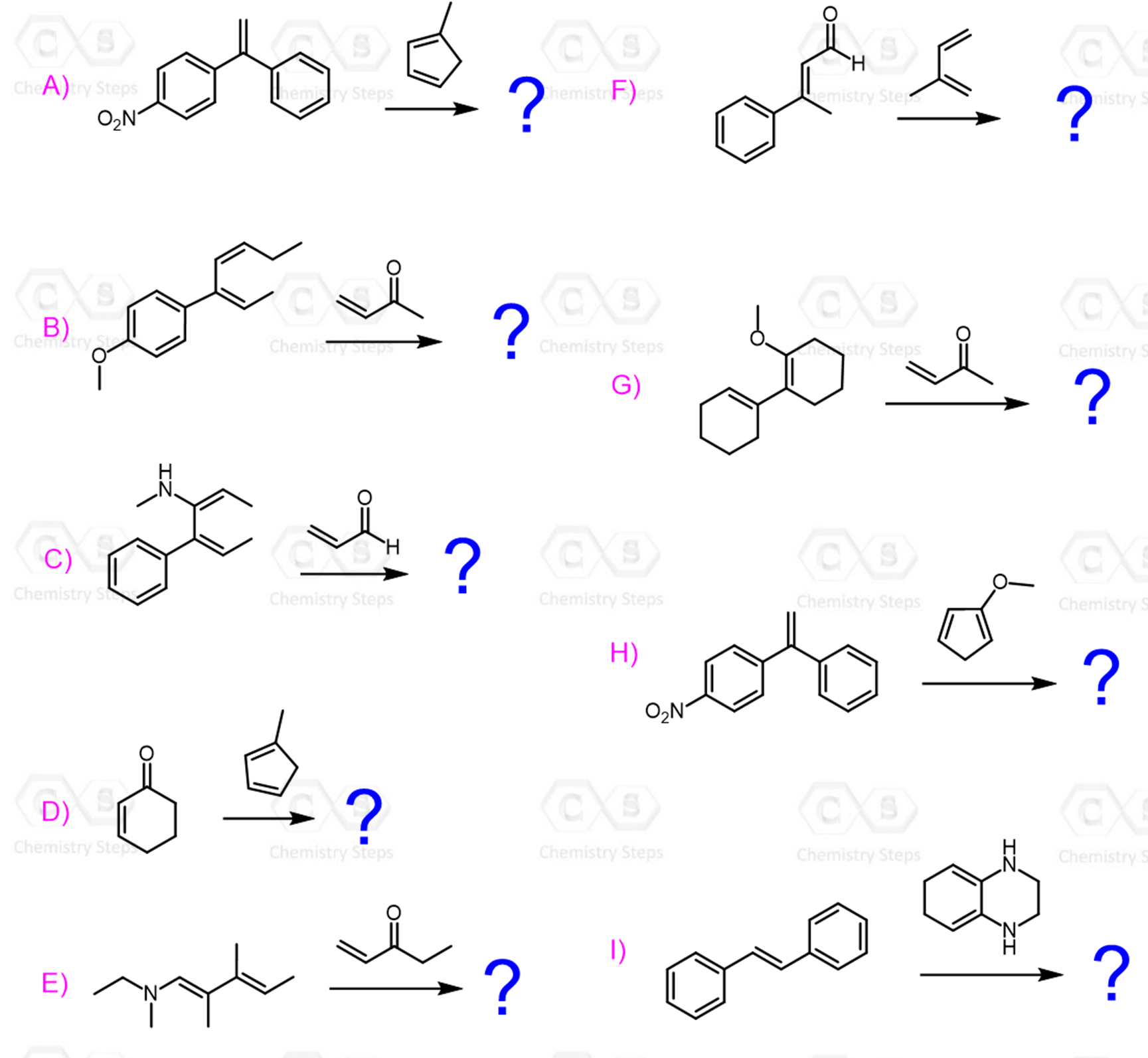
The Diels-Alder Reaction Practice Problems
For each Diels–Alder reaction, predict the major product(s) with correct stereochemistry when each cyclic diene is reacted with a dienophile:

Aromatic Substitution Practice Problems
Show how each compound can be synthesized from benzene and any other organic or inorganic reagents.
The order of reactions is very important! So, before every step, consider the ortho– , para– , or meta directing effect of the current group on the aromatic ring.
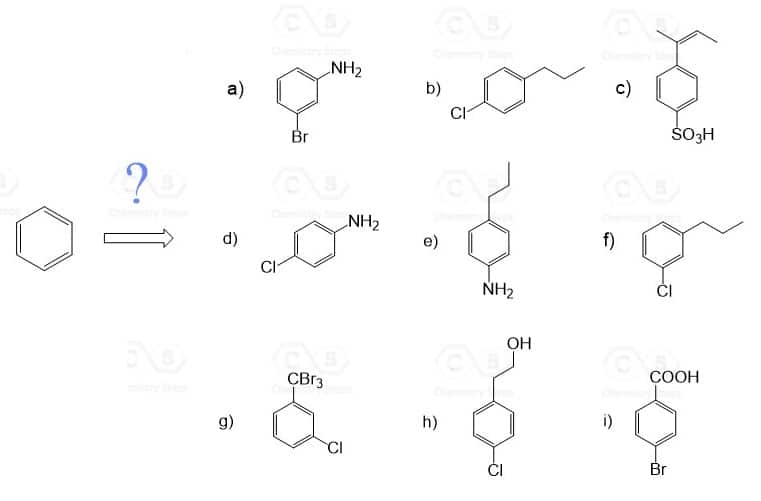
Devise a synthesis of each of the following compounds using an arene diazonium salt. They all require more than one step and you may select the desired regioisomer (for example the para product from an ortho, para mixture) when needed.

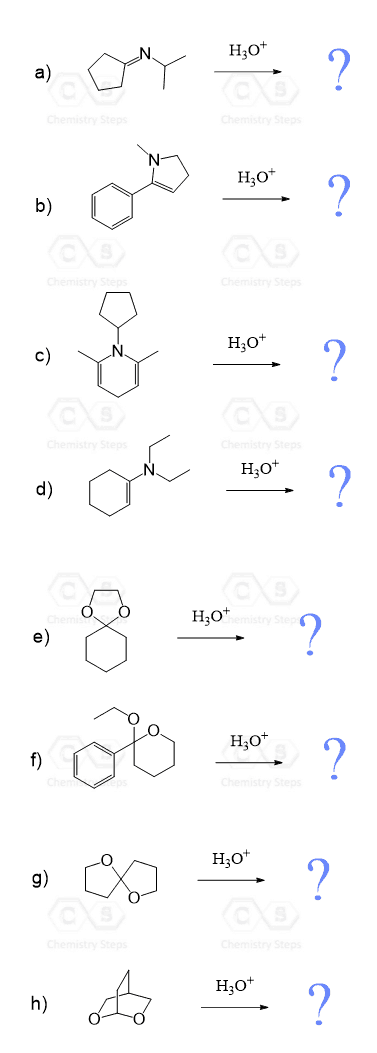
Aldehydes and Ketones Practice Problems
Predict the major product(s) obtained when each of the following compounds undergoes hydrolysis in the presence of an acid:
Carboxylic Acids and Their Derivatives Practice Problems
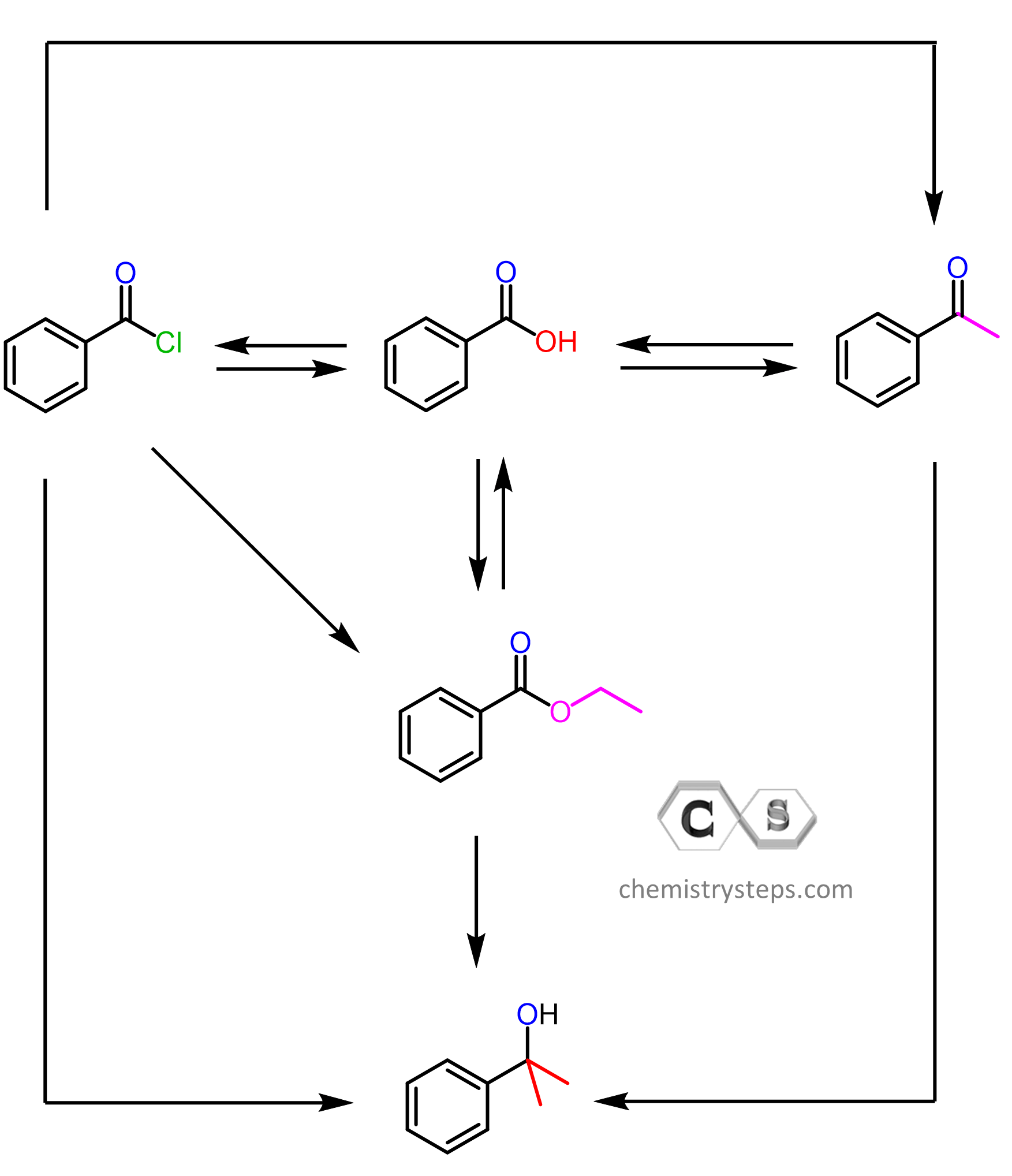
Predict the major organic product(s) for each of the following reactions. They all involve carboxylic acid derivatives such as esters, acid chlorides, nitriles, anhydrides, and amides. You may also need to go over the reactions covered in earlier chapters, particularly, the Grignard and Gilman reagents, oxidizing and reducing agents and electrophilic aromatic substitutions.
A link to each topic encountered in a given problem will be provided in the answer tab.

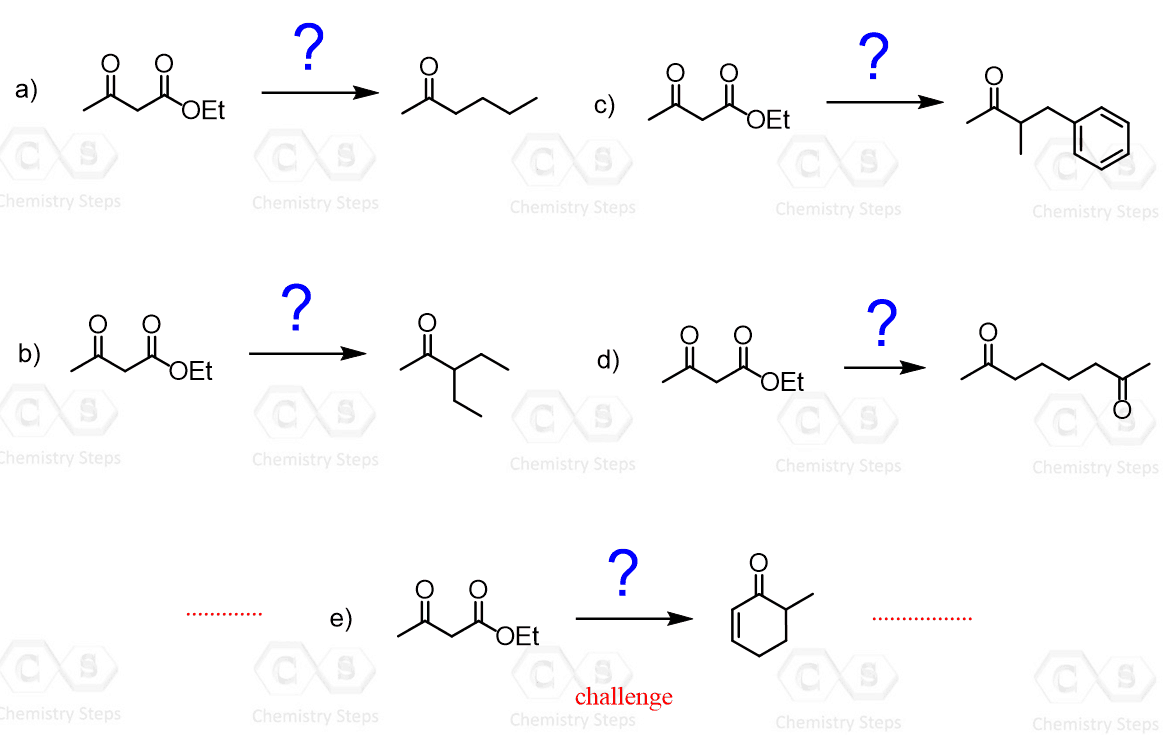
Alpha Carbon Chemistry – Enols and Enolates Practice Problems
This is a comprehensive practice problem on the alpha carbon chemistry. The topics covered range from the simple halogenation reactions of enols to multistep synthetic transformations.
To correctly answer these questions, you need to review the main principles of enolate chemistry – direct enolate alkylation, aldol condensation, crossed aldol condensation, alkylation using acetoacetic ester synthesis, malonic ester synthesis, the Stork enamine synthesis, Claisen condensation, Michael addition, and Robinson annulation.
Practice

Check out our new synthesis puzzles!
CS Prime membership will also grant you access to multiple-choice quizzes!
General Chemistry Overview Quiz
Molecular Representations Quiz
Organic Acids and Bases Quiz
Alkanes and Cycloalkanes Practice Quiz
Stereochemistry Practice Problems Quiz
Nucleophilic Substitution and Elimination Practice Quiz
Alkene Addition Reactions Practice Quiz
Alkyne Naming and Reactions Practice Quiz
Organic Chemistry Final Practice Quiz
Aromatic Compounds
Alcohols Quiz – Naming, Preparation, and Reactions
Aldehydes and Ketones Reactions Practice Quiz
Carboxylic Acids and Their Derivatives Quiz
Carbohydrates Practice Problem Quiz
IUPAC Nomenclature Summary Quiz
Table of Contents
Structure and Bonding
- Lewis Structures in Organic Chemistry
- Valency and Formal Charges in Organic Chemistry
- How to Determine the Number of Lone Pairs
- Bonding Patterns in Organic Chemistry
- sp3, sp2, and sp Hybridization in Organic Chemistry with Practice Problems
- How to Quickly Determine The sp3, sp2, and sp Hybridization
- Bond Lengths and Bond Strengths
- VSEPR Theory – Molecular and Electron Geometry of Organic Molecules
- Dipole-dipole, London Dispersion, and Hydrogen Bonding Interactions
- Dipole Moment and Molecular Polarity
- Boiling Point and Melting Point in Organic Chemistry
- Boiling Point and Melting Point Practice Problems
- Solubility of Organic Compounds
- General Chemistry Overview Quiz
Molecular Representations
- Bond-Line or Skeletal Structures
- Functional Groups in Organic Chemistry with Practice Problems
- Bond-line, Lewis, and Condensed Structures with Practice Problems
- Curved Arrows with Practice Problems
- Resonance Structures in Organic Chemistry with Practice Problems
- Rules for Drawing Resonance Structures
- Bonding Patterns in Organic Chemistry
- Major and Minor Resonance Contributor
- Significant Resonance Structures
- How to Choose the More Stable Resonance Structure
- Drawing Complex Patterns in Resonance Structures
- Localized and Delocalized Lone Pairs with Practice Problems
- Molecular Representations Quiz
Acids and Bases
- Acids and Bases – General Chemistry
- Organic Acids and Bases
- Organic acid-base mechanisms
- Acid Strength and pKa
- How to Determine the Position of Equilibrium for an Acid-Base Reaction
- Inductive and Resonance (Mesomeric) Effects
- Factors That Determine the pKa and Acid Strength
- How Resonance Affects Acidity and Basicity
- How to Choose an Acid or a Base to Protonate or Deprotonate a Given Compound
- Lewis Acids and Bases
- Basicity of Amines
- Organic Acids and Bases Practice Problems
- Organic Acids and Bases Quiz
Alkanes and Cycloalkanes
- Naming Alkanes by IUPAC Nomenclature Rules Practice Problems
- Naming Bicyclic Compounds
- Naming Bicyclic Compounds-Practice Problems
- How to Name a Compound with Multiple Functional Groups
- Primary, Secondary, and Tertiary Carbon Atoms in Organic Chemistry
- Constitutional or Structural Isomers with Practice Problems
- Degrees of Unsaturation or Index of Hydrogen Deficiency
- The Wedge and Dash Representation
- Sawhorse Projections
- Newman Projections with Practice Problems
- Staggered and Eclipsed Conformations
- Conformational Isomers of Propane
- Newman Projection and Conformational Analysis of Butane
- Newman Projection of Chair Conformation
- Gauche Conformation
- Gauche Conformation, Steric, Torsional Strain Energy Practice Problems
- Ring Strain
- Steric vs Torsional Strain
- Conformational Analysis
- Drawing the Chair Conformation of Cyclohexane
- Ring Flip: Drawing Both Chair Conformations with Practice Problems
- 1,3-Diaxial Interactions and A value for Cyclohexanes
- Ring-Flip: Comparing the Stability of Chair Conformations with Practice Problems
- Cis and Trans Decalin
- IUPAC Nomenclature Summary Quiz
- Alkanes and Cycloalkanes Practice Quiz
Stereochemistry
- How to Determine the R and S Configuration
- The R and S Configuration Practice Problems
- What is Nonsuperimposable in Organic Chemistry
- Chirality and Enantiomers
- Diastereomers-Introduction and Practice Problems
- Enantiomers vs Diastereomers
- Cis and Trans Isomers
- E and Z Alkene Configuration with Practice Problems
- Enantiomers vs Diastereomers
- Enantiomers, Diastereomers, the Same or Constitutional Isomers with Practice Problems
- Configurational Isomers
- Optical Activity
- Specific Rotation
- Racemic Mixtures
- Enantiomeric Excess (ee): Percentage of Enantiomers from Specific Rotation with Practice Problems
- Symmetry and Chirality. Meso Compounds
- Fischer Projections with Practice Problems
- R and S Configuration in the Fischer Projection
- R and S configuration on Newman projections
-
Converting Bond-Line, Newman Projection, and Fischer Projections
- Resolution of Enantiomers: Separate Enantiomers by Converting to Diastereomers
- Stereochemistry Practice Problems
- Stereochemistry Practice Quiz
Energy Changes In Organic Chemistry
- Energy and Organic Chemistry Reactions
- Bond Lengths and Bond Strengths
- Homolytic and Heterolytic Bond Cleavage
- The Heat of Reaction from Bond Dissociation Energies
Nucleophilic Substitution Reactions
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- Steric Hindrance in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
Alkenes: Structure, Stability, and Nomenclature
- Alkenes: Structure and Stability
- Naming Alkenes by IUPAC Nomenclature Rules
- Cis and Trans Isomers
- E and Z Alkene Configuration with Practice Problems
Elimination Reactions
- Substitution and Elimination Reactions
- The E2 Mechanism
- E2 Elimination Practice Problems
- Zaitsev’s Rule – Regioselectivity of E2 Elimination Reactions
- The Hofmann Elimination of Amines and Alkyl Fluorides
- Stereoselectivity of E2 Elimination Reactions
- Stereospecificity of E2 Elimination Reactions
- SN2 and E2 Rates of Cyclohexanes
- Elimination Reactions of Cyclohexanes with Practice Problems
- POCl3 for Dehydration of Alcohols
- The E1 Mechanism with Practice Problems
- Regioselectivity of E1 Reactions
- Stereoselectivity of E1 Reactions
- How to tell if it is E2 or E1 Mechanism
- SN1 vs E1 Reactions
- SN2 vs E2 Reactions
- Dehydration of Alcohols by E1 and E2 Elimination
- Mesylates and Tosylates as Good Leaving Groups
- Mitsunobu Reaction
- SN1 SN2 E1 E2 – How to Choose the Mechanism
- Polar Protic and Polar Aprotic Solvents
- SN1 SN2 E1 or E2 – the Largest Collection of Practice Problems
- The Hammond Postulate
- The E1cB Elimination Mechanism
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
Addition Reactions of Alkenes
- Electrophilic Addition Reactions to Alkenes
- Markovnikov’s Rule
- Markovnikov’s Rule with Practice Problems
- Addition of Water to Alkenes
- Acid-Catalyzed Hydration of Alkenes with Practice Problems
- Rearrangements in Alkene Addition Reactions
- Oxymercuration-Demercuration
- Addition of Alcohols to Alkenes
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Hydroboration-Oxidation: The Mechanism
- Hydroboration-Oxidation of Alkenes: Regiochemistry and Stereochemistry with Practice Problems
- Halogenation of Alkenes and Halohydrin Formation
- The Regiochemistry of Alkene Addition Reactions
- The Stereochemistry of Alkene Addition Reactions
- Cis product in an anti-Addition Reaction of Alkenes
- Ozonolysis of Alkenes with Practice Problems
- Syn Dihydroxylation of Alkenes with KMnO4 and OsO4
- Anti-Dihydroxylation of Alkenes with MCPBA and Other Peroxides with Practice Problems
- Oxidative Cleavage of Alkenes with KMno4 and O3
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkene Addition Reactions Practice Quiz
- Reactions Map of Alkenes
- Introduction to Alkynes
- Naming Alkynes by IUPAC Nomenclature Rules – Practice Problems
- Preparation of Alkynes by Elimination Reactions
- Hydrohalogenation of Alkynes
- Addition of Water to Alkynes
- Acid-Catalyzed Hydration of Alkynes with Practice Problems
- Reduction of Alkynes
- Halogenation of Alkynes
- Hydroboration-Oxidation of Alkynes with Practice Problems
- Ozonolysis of Alkynes with Practice Problems
- Alkylation of Terminal Alkynes in Organic Synthesis with Practice Problems
- Reactions of Acetylide Ions
- Alkyne reactions summary practice problems
- Alkyne Synthesis Reactions Practice Problems
- Alkyne Naming and Reactions Practice Quiz
- Reactions Map of Alkynes
Nuclear Magnetic Resonance (NMR) Spectroscopy
- NMR Spectroscopy – An Easy Introduction
- NMR Chemical Shift
- NMR Chemical Shift Range and Value Table
- NMR Number of Signals and Equivalent Protons
- Homotopic, Enantiotopic, Diastereotopic, and Heterotopic
- Homotopic Enantiotopic Diastereotopic Practice Problems
- Integration in NMR Spectroscopy
- Splitting and Multiplicity (N+1 rule) in NMR Spectroscopy
- NMR Signal Splitting N+1 Rule Multiplicity Practice Problems
- 13C Carbon NMR
- DEPT NMR: Signals and Problem Solving
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems
Organic Structure Determination
- NMR Spectroscopy-Carbon-Dept-IR Practice Problems
- Interpreting IR Spectra
- Infrared (IR) Spectroscopy Practice Problems
Radical Reactions
- Free-Radical Addition of HBr: Anti-Markovnikov Addition
- Initiation, Propagation, Termination in Radical Reactions
- Selectivity in Radical Halogenation
- Stability of Radicals
- Resonance Structures of Radicals
- Stereochemistry of Radical Halogenation with Practice Problems
- Allylic Bromination by NBS with Practice Problems
- Radical Halogenation in Organic Synthesis
Reactions of Alcohols
- Nomenclature of Alcohols: Naming Alcohols based on IUPAC Rules with Practice Problems
- Preparation of Alcohols via Substitution or Addition Reactions
- Reaction of Alcohols with HCl, HBr, and HI Acids
- Mesylates and Tosylates as Good Leaving Groups
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in Substitution Reactions Practice Problems
- POCl3 for Dehydration of Alcohols
- Dehydration of Alcohols by E1 and E2 Elimination
- The Oxidation States of Organic Compounds
- LiAlH4 and NaBH4 Carbonyl Reduction Mechanism
- Alcohols from Carbonyl Reductions – Practice Problems
- Grignard Reaction in Preparing Alcohols with Practice Problems
- Grignard Reaction in Organic Synthesis with Practice Problems
- Protecting Groups For Alcohols in Organic Synthesis
- Oxidation of Alcohols: PCC, PDC, CrO3, DMP, Swern, and All of That
- Diols: Nomenclature, Preparation, and Reactions
- NaIO4 Oxidative Cleavage of Diols
- The Pinacol Rearrangement
- The Williamson Ether Synthesis
- Alcohol Reactions Practice Problems
- Naming Thiols and Sulfides
- Reactions of Thiols
- Alcohols Quiz – Naming, Preparation, and Reactions
- Reactions Map of Alcohols
Ethers and Epoxides
Conjugated Systems
- Resonance and Conjugated Dienes
- Allylic Carbocations
- 1,2 and 1,4 Electrophilic Addition to Dienes
- Kinetic vs Thermodynamic Control of Electrophilic Addition to Dienes
The Diels-Alder Reaction
- Diels-Alder Reaction: Dienes and Dienophiles
- Predict the Products of the Diels-Alder Reaction with Practice Problems
- Endo and Exo Products of Diels-Alder Reaction with Practice Problems
- Regiochemistry of the Diels–Alder Reaction with Practice Problems
- Identify the Diene and Dienophile of the Diels-Alder reaction with Practice Problems
- Diels-Alder Reaction in Organic Synthesis Practice Problems
Aromatic Compounds
- Naming Aromatic Compounds
- Introduction to Aromatic Compounds
- Benzene – Aromatic Structure and Stability
Aromaticity and Huckel’s Rule - The 4n+2 Rule
- Identify Aromatic, Antiaromatic, or Nonaromatic Compounds
- Frost Circle
- Annulenes
Electrophilic Aromatic Substitution
- Electrophilic Aromatic Substitution – The Mechanism
- The Halogenation of Benzene
- The Nitration of Benzene
- The Sulfonation of Benzene
- Activating and Deactivating Groups
- Friedel-Crafts Alkylation with Practice Problems
- Friedel-Crafts Acylation with Practice Problems
- Vilsmeier-Haack Reaction
- The Alkylation of Benzene by Acylation-Reduction
- Ortho, Para, Meta in EAS with Practice Problems
- Ortho, Para, and Meta in Disubstituted Benzenes
- Why Are Halogens Ortho-, Para- Directors yet Deactivators?
- Is Phenyl an Ortho/Para or Meta Director?
- Limitations of Electrophilic Aromatic Substitution Reactions
- Orientation in Benzene Rings With More Than One Substituent
- Synthesis of Aromatic Compounds From Benzene
- Arenediazonium Salts in Electrophilic Aromatic Substitution
- Reactions at the Benzylic Position
- Benzylic Bromination
- Nucleophilic Aromatic Substitution
- Nucleophilic Aromatic Substitution Practice Problems
- Reactions of Phenols
- Reactions of Aniline
- Meta Substitution on Activated Aromatic Ring
- Electrophilic Aromatic Substitution Practice Problems
- Aromatic Compounds Quiz
- Reactions Map of Aromatic Compounds
Aldehydes and Ketones
- Nomenclature of Aldehydes and Ketones
- How to Name a Compound with Multiple Functional Groups
- Preparation of Aldehydes and Ketones
- Nucleophilic Addition to Carbonyl Groups
- Reduction of Aldehydes and Ketones
- Reactions of Aldehydes and Ketones with Water
- Reactions of Aldehydes and Ketones with Alcohols: Acetals and Hemiacetals
- Acetals as Protecting Groups for Aldehydes and Ketones
- Formation and Reactions of Imines and Enamines
- Reductive Amination
- Acetal Hydrolysis Mechanism
- Imine and Enamine Hydrolysis Mechanism
- Hydrolysis of Acetals, Imines, and Enamines-Practice Problems
- Reaction of Aldehydes and Ketones with CN, Cyanohydrin Formation
- The Wittig Reaction: Examples and Mechanism
- The Wittig Reaction: Practice Problems
- Aldehydes and Ketones to Carboxylic Acids
- Reactions of Aldehydes and Ketones – Practice Problems
- Aldehydes and Ketones Reactions Practice Quiz
- Reactions Map of Aldehydes
- Reactions Map of Ketones
Carboxylic Acids and Their Derivatives-Nucleophilic Acyl Substitution
- Preparation of Carboxylic Acids
- Naming Carboxylic Acids
- Naming Nitriles
- Naming Esters
- Naming Carboxylic Acid Derivatives – Practice Problems
- The Addition-Elimination Mechanism
- Fischer Esterification
- Ester Hydrolysis by Acid and Base-Catalyzed Hydrolysis
- What is Transesterification?
- Esters Reaction with Amines – The Aminolysis Mechanism
- Ester Reactions Summary and Practice Problems
- Preparation of Acyl (Acid) Chlorides (ROCl)
- Reactions of Acid Chlorides (ROCl) with Nucleophiles
- R2CuLi Organocuprates – Gilman Reagent
- Reaction of Acyl Chlorides with Grignard and Gilman (Organocuprate) Reagents
- Reduction of Acyl Chlorides by LiAlH4, NaBH4, and LiAl(OtBu)3H
- Reduction of Carboxylic Acids and Their Derivatives
- Preparation and Reaction Mechanism of Carboxylic Anhydrides
- Amides – Structure and Reactivity
- Naming Amides
- Amides Hydrolysis: Acid and Base-Catalyzed Mechanism
- Amide Dehydration Mechanism by SOCl2, POCl3, and P2O5
- Amide Reduction Mechanism by LiAlH4
- Reduction of Amides to Amines and Aldehydes
- Amides Preparation and Reactions Summary
- Amides from Carboxylic Acids-DCC and EDC Coupling
- The Mechanism of Nitrile Hydrolysis To Carboxylic Acid
- Nitrile Reduction Mechanism with LiAlH4 and DIBAL to Amine or Aldehyde
- The Mechanism of Grignard and Organolithium Reactions with Nitriles
- The Reactions of Nitriles
- Converting Nitriles to Amides
- Carboxylic Acids to Ketones
- Esters to Ketones
- Carboxylic Acids and Their Derivatives Practice Problems
- Carboxylic Acids and Their Derivatives Quiz
- Reactions Map of Carboxylic Acid Derivatives
Alpha Carbon Chemistry: Enols and Enolates
- Keto-Enol Tautomerization
- Alpha Halogenation of Enols and Enolates
- The Haloform and Iodoform Reactions
- Alpha Halogenation of Carboxylic Acids
- Alpha Halogenation of Enols and Enolates Practice Problems
- The E1cB Elimination Mechanism
- Aldol Reaction – Principles and Mechanism
- Aldol Condensation – Dehydration of Aldol Addition Product
- Intramolecular Aldol Reactions
- Aldol Addition and Condensation Reactions – Practice Problems
- Crossed Aldol And Directed Aldol Reactions
- Crossed Aldol Condensation Practice Problems
- The Cannizzaro reaction
- Alkylation of Enolates Alpha Position
- Enolate Alkylation Practice Problems
- Acetoacetic Ester Synthesis
- Acetoacetic Ester Enolates Practice Problems
- Malonic Ester Synthesis
- Decarboxylation
- Michael Reaction: The Conjugate Addition of Enolates
- Robinson Annulation, Shortcut, and Retrosynthesis
- Claisen Condensation
- Dieckmann Condensation – An Intramolecular Claisen Reaction
- Crossed Claisen and Claisen Variation Reactions
- Claisen Condensation Practice Problems
- Stork Enamine Synthesis
- Mannich Reaction
- Enolates in Organic Synthesis – a Comprehensive Practice Problem
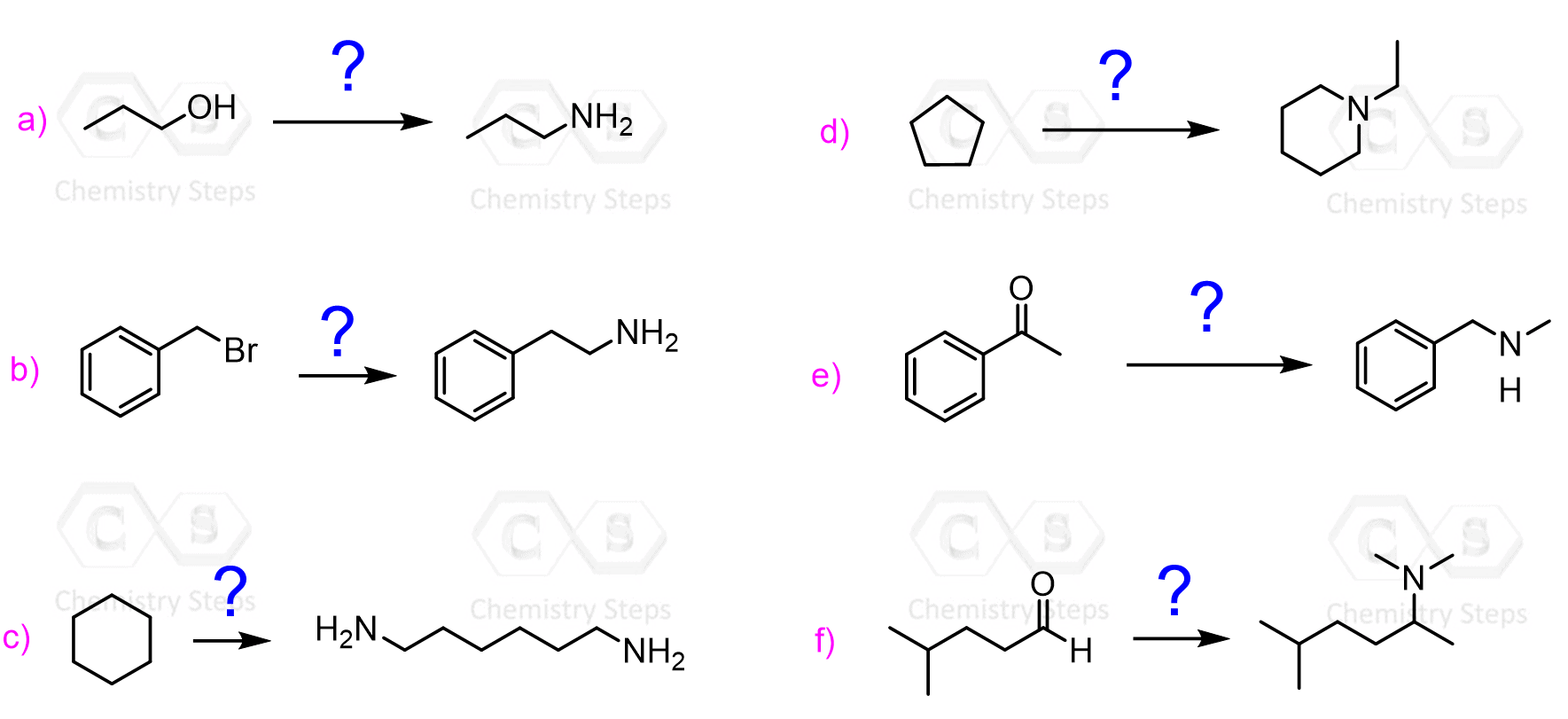
Amines
- Naming Amines: Systematic and Common Nomenclature
- Preparation of Amines
- The Gabriel Synthesis of Primary Amines
- Imines from Aldehydes and Ketones with Primary Amines
- Enamines from Aldehydes and Ketones with Secondary Amines
- The Hofmann Elimination of Amines and Alkyl Fluorides
- The Reaction of Amines with Nitrous Acid
- Reactions of Amines Practice Problems
- The Cope elimination
- Basicity of Amines
- Boc Protecting Group for Amines
Organic Synthesis Problems
- Organic Chemistry Multistep Synthesis Practice Problems
- Organic Synthesis Puzzles
- Acetals as Protecting Groups for Aldehydes and Ketones
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Alkene Reactions Practice Problems
- Changing the Position of a Double Bond
- Changing the Position of a Leaving Group
- Alkenes Multi-Step Synthesis Practice Problems
- Alkyne Synthesis Reactions Practice Problems
- Radical Halogenation in Organic Synthesis
- Grignard Reaction in Organic Synthesis with Practice Problems
- Ortho Para Meta in EAS with Practice Problems
- Orientation in Benzene Rings With More Than One Substituent
Carbohydrates
- Carbohydrates – Structure and Classification
- Erythro and Threo
- R and S Configuration on Fischer Projections
- D and L Sugars
- Aldoses and Ketoses: Classification and Stereochemistry
- Epimers and Anomers
- Converting Fischer, Haworth, and Chair forms of Carbohydrates
- Mutarotation
- Glycosides
- Isomerization of Carbohydrates
- Ether and Ester Derivatives of Carbohydrates
- Oxidation of Monosaccharides
- Reduction of Monosaccharides
- Kiliani–Fischer Synthesis
- Wohl Degradation
- Carbohydrates Practice Problem Quiz
All the practice problems are open to everyone for free! I have seen and prepared hundreds of exams for organic chemistry, and these practice problems are the types that you will find in your exams. Their difficulty varies from one-step to more advanced ones, including step organic synthesis problems.
After working on each question, you have the possibility to first check your answer and then refer to the step-by-step solution.
The practice problems are the only ones you get to do.
Multiple-choice quizzes might be the “easy” way of glancing through the key concepts and getting feedback on what you need to work on more.
These are also included in your Chemistry Steps membership.